ASCIDIAN NEWS*
Gretchen
Lambert
12001 11th
Ave. NW, Seattle, WA 98177
206-365-3734 gretchen.lambert00@gmail.com
home page:
http://depts.washington.edu/ascidian/
Number
74 December 2014
Rosana Rocha and
I taught a two week workshop June 17-July 2 at the Smithsonian Tropical
Research Institute (STRI) at Bocas del Toro, Panama. This was the 4th ascidian
workshop at Bocas; the first was in 2006. It is very gratifying to see that
many of today’s biologists working on ascidians have taken our workshops over
the years. In July Susanna López-Legentil (Univ. of N. Carolina) and
I conducted an ascidian survey of many marinas and some aquaculture sites along
the North Carolina coast, a region that has not been surveyed for ascidians for
many decades. See the abstract below.
I greatly
enjoyed seeing many friends at the 5th Intl. Invasive Sea Squirt
Conference in Woods Hole, Mass. Oct. 29-31. The talks and posters (see the link
to the full program, including abstracts, below in the Abstracts from Recent
Meetings section) were very interesting yet also disturbing to realize that
invasive ascidians are a continuing and indeed increasingly big problem
worldwide.
*I draw your attention to an important
contribution in the Work in Progress section, by John Ryland, on finally
elucidating the proper gender of Botrylloides
(masculine). This is going to mean changing the ending on a number of species
in this genus; please read.
There are 124 new publications listed at the end
of this newsletter. With all the interest in Ciona A and B species, see the Sato et al. 2014 reference.
*Ascidian News is not part of the scientific
literature and should not be cited as such.
NEWS AND VIEWS
1. From Gaku Kumano:
The 8th Intl.
Tunicata meeting will be held on July 13-17, 2015 in Aomori city, Japan. Aomori
city is located at the northern end of the Japan main island, being surrounded
by well-known scenic places such as the Shirakami Mountains World Heritage Site
and Lake Towada. July in Aomori is a pleasant season as the temperature
averages approximately 22o C. The organizers are Kazuo Inaba,
Director, Shimoda Marine Research Center, University of Tsukuba (inaba@kurofune.shimoda.tsukuba.ac.jp) and Gaku Kumano, Asamushi Research Center for Marine Biology, Tohoku
University, Asamushi (kumano@m.tohoku.ac.jp).
A website has been uploaded at http://tunicatemeeting.info/Aomori2015/. Resistration and abstract submission
is scheduled to start on April 1, 2015. Please visit the website and we look
forward to seeing you all at the meeting.
2. From Emma
Johnston: The 9th Intl. Conference on Marine Bioinvasions will be held in Sydney, Australia from the 19-21st of January 2016. e.johnston@unsw.edu.au
3. From Hitoshi
Sawada (hsawada@bio.nagoya-u.ac.jp):
a) Dr. Sawada received a Zoological Society Award September 12, 2014 for: "Studies on the Mechanisms of Ascidian Fertilization". Our congratulations, Hitoshi!
b) Drs. Maki Shirae-Kurabayashi, Yuji Ise,
and Shiori Nakazawa joined the Sugashima Marine Biological Laboratory as
Designated Assistant Professors April 1, 2014. Mainly by these members, the
International Summer Course for graduate and undergraduate students will be
organized next July, 2015, at the Sugashima MBL. The course will deal with
genome editing techniques, and proteomic and molecular phylogenetic analyses,
using ascidians and sponges. Detailed information will be up-loaded to the
following website.
http://www.bio.nagoya-u.ac.jp/~SugashimaMBL/index-en.html
Those who are interested in attending this course,
please send an e-mail to Hitoshi Sawada (hsawada@bio.nagoya-u.ac.jp). Partial financial support for
traveling expense will be considered upon request.
4. From Gretchen Lambert: Dr. Shigeko Ooishi,
a world-renowned taxonomist of a specialized group of small crustaceans,
copepods parasitic in ascidians, died on September 14 at age 87, after a long
and illustrative career. She was born in 1927 in Kumamoto, Japan, the second of
four children. Her
parents ran a popular buckwheat noodle restaurant for many years; patrons
included famous politicians and writers. Shigeko earned her
baccalaureate at what is now known as Nara Women’s University, and joined the
Faculty of Fisheries at Mie University in 1951 as an assistant. She remained at
Mie Univ. for 40 years, rising to the position of professor after receiving her
doctorate in 1965 from Nagoya University on decapod embryology; she
published a number of papers on various aspects of crustacean biology during
her many years at Mie University. My husband Charley and I and
our daughters greatly enjoyed a visit with her in Mie in 1992. Mie is
located in an important region of pearl culture, and for many years Shigeko’s
teaching duties included training hundreds of students on aspects of pearl
culture. During the
1970’s and 1980’s Shigeko spent many summers at the Friday Harbor
Laboratories working with Dr. Paul Illg on the systematics and biology of
parasitic copepods. After her retirement from Mie Univ., she
moved to FHL where she continued her work for 23 years, until May of this year
when she moved back to Japan. She
published many papers and monographs: 38 on parasitic copepods, 5 of them with
Paul Illg. She traveled widely to collect and photograph live copepods
living in ascidians, in order to record their unique color patterns, including
Roscoff in Brittany, France; Portobello Marine Lab in southern New Zealand;
Hopkins Marine Lab in Pacific Grove, CA. Her last work was ascidian-associated
copepods of Arthur Humes collections from Madagascar;
she completed the last two papers just before returning to Japan. In 2013 she was named an
Honorary Fellow of the E.S. Morse Institute (see article and photo in Fall 2013 FHL newsletter).
I will greatly miss Shigeko; we were friends for many decades. Tributes can be made in memory of Shigeko to any of the FHL scholarship funds (http://depts.washington.edu/fhl/help_endowments.html ), several of which she supported for many years, especially the Patricia L. Dudley Endowment for Friday Harbor Laboratories.
-----With
help from Dr. Keiji Baba (kbaba.kumamoto@gmail.com) and information from: History of Carcinology edited by
Frank Truesdale, 1993. CRC Press. Chapter 6: Women’s contributions
to carcinology by P.A. McLaughlin and S. Gilchrist. Pp. 196-197: A woman carcinologist in Japan: Shigeko Ooishi.
5. From Christian Sardet, Emeritus, CNRS Villefranche, csardet@gmail.com
: The American
edition of my book "Plancton - aux origines du vivant" (Ulmer
2013) will be published in April 2015 by University of Chicago Press under
the title "Plankton - Wonders of
The Drifting World" http://www.press.uchicago.edu/ucp/books/book/chicago/P/bo19415930.html
In France the book has been very
well-received, and recently obtained a prize as "best underwater world
guide":
http://www.editions-ulmer.fr/editions-ulmer/plancton-aux-origines-du-vivant-362-cl.htm
It was recently published in Japanese:
http://www.kawade.co.jp/np/isbn/9784309253084/
6. From Stefano Tiozzo, Observatoire Océanologique de Villefranche-sur-Mer, France :
We are recruiting researchers at
different levels to join a 4-years international project, DEVODIVERSITY, funded
by the French Agence Nationale de la Recherché (ANR) and the São Paulo Research
Foundation (FAPESP).
The Brown Lab at the Instituto de Biociências
in Brazil (USP) and the Tiozzo Lab at the Villefranche-sur-Mer Developmental Biology
Laboratory in France (CNRS-UPMC) will study the evolution of regeneration,
asexual reproduction, and clonality in several species of ascidians
(Urochordata), and examine how ecological factors affect distribution ranges,
evolution of life cycles, and developmental strategies.
DEVODIVERSITY has the
following aims:
1.
To resolve the phylogenetic relationships and evolutionary transitions between
strictly sexual reproduction to budding or high regenerative abilities among
Styelidae (Ascidiacea) species.
2.
To provide a morphological and ecological understanding of asexual propagation
(budding). We will generate detailed anatomical and developmental descriptions
of budding processes, and explore if environmental conditions are associated
with the use of particular budding modes.
3.
To compare gene pathways involved in cell function or trans/de-differentiation
processes of budding and regeneration by in silico analysis of
transcriptomic data.
4. To launch a comparative
genomic approach if styelid ascidians to better understand the evolution of
major life history transitions in marine chordates, in particular the
evolutionary transition from sexual to asexual propagation.
For more information, contact Stefano Tiozzo tiozzo@obs-vlfr.fr or Federico Brown fdbrown@usp.br .
WORK
IN PROGRESS
1. From Don Deibel, Fisheries
and Oceans Canada, Northwest Atlantic Fisheries Centre,
St. John’s,
Newfoundland, Canada ddeibel@mun.ca :
Deibel, D., McKenzie, C.H., Rise, M.L., Thompson,
R.J., Lowen, J.B., Ma, K.C.K., Applin, G., O’Donnell, R., Wells, T., Hall,
J.R., Sargent, P., and Pilgrim, B.B. 2014. Recommendations for
eradication and control of non-indigenous, colonial ascidian tunicates in
Newfoundland harbours. Canadian Manuscript Reports of Fisheries and Aquatic Sciences. 3039: xi + 60 p. The pdf can
be downloaded from the website of the Department of Fisheries and Oceans
Canada: http://www.dfo-mpo.gc.ca/library/352814.pdf
2. From
Xavier Turon, Blanes, Spain xturon@ceab.csic.es : A review of
invasion genetics of marine organisms in Europe by Marc Rius, Xavier Turon,
Giacomo Bernardi, Filip Volckaert and Frédérique Viard [Rius et al. 2014, Biol.
Invasions; see New Publications at end of newsletter] has highlighted that
ascidians are the group for which more genetic studies on introduced species
have been published in the area. A detailed list of these studies is given in
the supplementary material, and general conclusions about genetic patterns of
non-indigenous species and evolutionary implications are drawn from the data
compiled.
3. From John Ryland j.s.ryland@swansea.ac.uk The gender of Botrylloides is masculine.
I have been revising the Tunicata (actually only Ascidiacea) chapter of
the Handbook of the Marine Fauna of North-West Europe (Hayward &
Ryland 1995) and realized that there is a problem with the gender of Botrylloides,
various species of which now occur around the British Isles. The genus contains
invasive species and is constantly in the news, so getting the gender right is
important. The ruling in the Code (Art. 30.1.4.4) is clear enough: a genus
group name ending –oides is masculine, “unless
its author, when establishing the name, stated that it had another gender or
treated it as such by combining it with an adjectival species-group name in
another gender form”.
The World Register of Marine Species (WoRMS) lists 19 species of Botrylloides
that are considered valid. Eight, such as B. aureum, B. chevalense, and
B. nigrum are neuter, nine are masculine including
B.violaceus, altered from Oka’s (1927) violaceum with the
annotation that the latter is an “incorrect original spelling”. This assertion
is not explained and is rather odd when a further eight have retained
their neuter gender.
Botrylloides
was introduced by H. Milne Edwards (1841) with four included species: B.
rotifera, B. rubrum and B.
albicans, all new (pp. 85–88), and B. leachii (Savigny) (p. 88). Additionally, Botrylloides
violaceus was used in the plate caption (p. 108). This is clearly a lapse for Botryllus
violaceus which, in the text, is
described under Botryllus “proprement dits” (strictly speaking) (p.89),
but still must be taken into account. The first specific name, rotifera, is the feminine of a properly
compounded Latin adjective rotifer, -fera, -ferum; derived from rota, a wheel, and fero,
ferre, to carry. The second name, rubrum,
is clearly neuter. Botrylloides violaceus,
whether a slip or not, implies a masculine gender. The contradiction of gender
between the first two included species was noted by Giard (1872): “M Edwards
en créant le mot Botrylloides
l’a fait du féminin ou du neutre. Si du neutre, pourquoi B. rotifera? et si du féminin, pourquoi B. rubrum?” The remaining
species, B. albicans and B. leachii, were not mentioned by Giard
(1872), presumably because they carry no indication of gender, but his account
leaves the unfortunate impression that only two species were included by Milne
Edwards (1841). So where does that leave
the gender of Botrylloides?
Ritter & Forsyth (1917) introduced Botrylloides diegensis (as masculine).
Oka (1927), a decade later, treated the genus as neuter, as did Van Name
(1945), including B. aureum Sars, B. nigrum Herdman, B.
diegense (altering the gender from Ritter & Forsyth), and B. magnum
(Ritter); so also did Tokioka (1953).
More recently, Kott (1985) took the same view, with B. magnicoecum
Hartmeyer, B. perspicuum Herdman, B. violaceum Oka, though she
later (1998) treated the genus as masculine, with B. perspicuus Herdman
(from B. perspicuum) and B. violaceus Oka (from B. violaceum). Why did she change her mind? In recent years, Japanese authors (Tokioka
1970, Saito et al. 1981) also have used the masculine form.
By introducing one species with feminine
gender and one with a neuter gender, Milne Edwards (1841) did not satisfy the
Code’s exception to its general rule “… that it had
another gender … combining it with an
adjectival species-group name in another gender form”. He left mixed signals
and for that reason the main provision of the Code (see above) applies. As with other genera ending in –oides, Botrylloides must be treated as masculine. The spelling of all extant species names
with neuter endings should be altered to masculine when opportunity arises; in
particular, uniformity should be applied throughout the list in WoRMS.
As stated by Kott (1985)
the type species of Botrylloides is B. rotifera, designated by Ärnbäck
Christie-Linde (1925). The use of a feminine adjective, rotifera, does not affect the conclusion that Botrylloides should have masculine gender.
My thanks are due to Miguel Alonso-Zarazaga, Daphne Fautin, Gretchen
Lambert, Gary Rosenberg and Judith Winston for their input.
References
Ärnbäck
Christie-Linde, A. (1925) On
the generic names Botrylloides Milne
Edwards and Metrocarpa Ärnbäck. Ark. Zool. 17b, No. 12, 1–6.
Giard, A.M. (1872) Récherches sur les
ascidies composées ou synascidies. Archs Zool. exp.
gén. 1:501-704
Hayward, P.J. &
Ryland, J.S. (1995) Handbook of the marine fauna of north-west Europe. Oxford University Press, Oxford, 800
pp.
Kott, P. (1985) The
Australian Ascidiacea Pt 1, Phlebobranchia and Stolidobranchia. Mem. Qld Mus., 23, 1–440.
Kott, P. (1998.) Tunicata.
In: Wells, A. & Houston, W.W.K. (Eds.) Zoological
Catalogue of Australia. Vol. 34. Hemichordata,
Tunicata, Cephalochordata. CSIRO Publishing, Melbourne, pp. 51–252,
259–261, 265–292.
Milne Edwards, H. (1841) Observations sur les
ascidies composées des côtes de la Manche, 1–110; (1842) Mem. Acad. Sci. Inst. Fr. 18:
217-326.
Oka, A. (1927) Zur
kenntniss der japanischen Botryllidae. Proc. imp. Acad.
Japan 3: 607-9
Ritter,
W. E. & Forsyth, R. A. 1917. Ascidians of the littoral
zone of southern California. U.C.
Publ. Zool. 16: 439-512.
Saito,
Y., Mukai, H. & Watanabe, H. (1981). Studies on Japanese compound styelid
ascidians II. A new species of the genus Botrylloides and redescription of B. violaceus Oka. Publ.
Seto Mar. Biol. Lab. 26:
357-368.
Tokioka, T. (1953). Ascidians of Sagami Bay. Iwanami
Shoten, Tokyo. 315 pp.
Tokioka,
T. (1970).
Contributions to Japanese ascidian fauna XXV. Notes on the variations in Botrylloides
violaceus Oka, with the description of a new subspecies tenuicoecus. Publ. Seto Mar. Biol. Lab. 18:
57-59.
Van Name, W.G. (1945). The North and South
American ascidians. Bull. Am. Mus. nat. Hist. 84:1-476.
World Register of Marine Species
(WoRMS) (Botrylloides 2004, 2007): www.marinespecies.org
4. From Gerard Breton, Le Havre, France gerard-breton@orange.fr :
Below
is a brief paper
reporting about an unusual appearance of a population of Didemnum vexillum in a basin of the port of Le Havre, in September
and October 2014.
On 18th September 2014, during a dive of the
association "Port Vivant" in a basin of the port of Le Havre (Eastern Channel, 49° 29' 20'' N; 0° 07'
07'' E), the so-called Bassin de la Barre, we noticed that the Didemnum vexillum, which were
rather abundant between the lowest water level and the foot of the quay wall on the quay wall that we explored,
were frequently affected by a curious morphological
modification, that we nicknamed "the Didemnum's balloon
disease".

The last picture on the right above, shows a "balloon" fallen on the sediment at
the foot of the quay wall and deflated.

The picture (left) shows a population of "normal" Dvex and "balloon" Dvex. Right: a "balloon" which does not collapse when dropped carefully on a glove. © Tierry Morin- Port Vivant.
Most of the colonies of Didemnum vexillum were inflated, "vesiculated", sometimes reaching a balloon shape of 1-6 cm in diameter. They are filled with seawater, not air or any gas. The openings of the "balloon" are in some cases the edge of the colony, in other cases, the common atrial siphons. Since the wall of the "balloon" is much thinner than the original colony, the color of the ballooned Dvex is from very pale yellowish to translucent greyish, and then, it look very much like some colonies of Diplosoma listerianum to naked eye. When carefully removed from the substrate, a Dvex
balloon keeps its spherical shape, but more often, it
collapses.
Two further dives, on 12th October 2014 and 24th October 2014, in the same basin, allowed to
further define the extension of the "disease", the ballooned Dvex
being present on nearly all substrates, quay walls, rocks, pontoons, between the lowest water level
and the deepest - 7 m, regardless of the orientation, of the depth, in the 0.7 km or so explored quays and
rocks. The vesiculated and
"ballooned" colonies are the most frequent, but some ones have a "normal" yellowish
color, with a firm "normal"
consistency. They are scattered among the ballooned or vesiculated grey
Dvex, and are very distinct. They seem to be, in average, nearer the surface. They are thought to be more recent  colonies. Very
rarely, we have met colonies with vesiculated parts and normal parts, but this may be due to an
undetectableinterpenetration of two
different colonies.
colonies. Very
rarely, we have met colonies with vesiculated parts and normal parts, but this may be due to an
undetectableinterpenetration of two
different colonies.
The colony in the center of the picture shows a vesiculated
part and a "normal" part. © Thierry Derycke.
All pictures
but two © Gérard Breton - Port Vivant Gérard Breton, Association Port Vivant, Le
Havre
14th November
2014
I do not know what to do with this observation,
it just reports field observations. Have any AN
readers seen something like this? Francoise Monniot told me that she had seen
it once, in the port of La Rochelle. I have collected specimens of both balloon
and normal D. vexillum in alcohol and
in sea water + formalin, if anyone wishes to examine or sequence a sample.
ABSTRACTS FROM RECENT
MEETINGS
1. XVIII Iberian
Marine Biology Symposium, Gijón, Spain, 2-5 September 2014.
The life
and times of the introduced ascidian Styela plicata: Pathway to a
holistic understanding. M. C.
Pineda1,2, S. Lopez-legentil2,3,
X. Turon4
1Australian Inst. of Mar. Sci., Townsville,
Queensland, Australia mcarmen.pineda@gmail.com
2Departament de Biologia Animal, Universitat de
Barcelona, Spain
3Center for Mar. Sci., Univ. of North Carolina,
Wilmington, USA LopezLegentils@uncw.edu
Center for
Advanced Studies of Blanes, Spain xturon@ceab.csic.es
Styela plicata is a solitary ascidian introduced all
around the world by ship traffic and seems to have many of the required
features to become invasive. We aimed to summarize here the knowledge acquired
about this species during the last years and to pinpoint the pathway for
further research. The global genetic composition of this species, its genetic
temporal structure, its reproductive features, the bacterial composition of its
tunic, and its capacity to cope with stress during early life-history stages
and adulthood have been recently assessed. Results indicate that S. plicata
is an ancient introduced species that has been travelling around the globe
through maritime transport for centuries. It inhabits harbours, marinas and
artificial structures, tolerating high concentration of pollutants. In these
habitats the species is a pest for bivalve cultures and a nuisance for
infrastructure maintenance. Further, expansion to neighbouring natural
environments should be regarded as a serious potential threat. Adults can
respond to moderate levels of stressors by adjusting the production of
stress-related proteins, but early stages are comparatively much more
vulnerable to the harsh conditions that characterize the habitats where this
species thrives. A prolonged reproductive period allows S. plicata to
exploit temporal windows of favourable conditions and confers to it a
competitive advantage compared to organisms with limited, seasonal reproduction
events. Bacterial communities in S. plicata have been reported to be
dynamic and could have the potential to aid host acclimation to new habitats by
establishing relationships with beneficial, locally sourced bacteria. In
addition, high genetic variability and the continual presence of larvae also
guarantee further reintroduction events and spreading via ship traffic. At
present, the distribution of S. plicata appears to be limited by high
temperature and low salinities. However, further studies are required to
determine other relevant factors regulating the spread of this species outside
enclosed environments (e.g. competition, predation, hydrodynamics), and to
understand the dynamics of the few populations co-habiting with native
communities, in order to design adequate management and eradication plans
should this species spread and become a threat to local biota.
2. 75TH
Natl. Conference of the Unione Zoologica Italiana, Bari, September 22-25, 2014
Lectins and immunity in compound
ascidians. Nicola Franchi, Filippo Schiavon, Loriano Ballarin, Department
of Biology, University of Padova ballarin@bio.unipd.it
Lectins are proteins able to recognize and
bind specific glycoconjugates, widely distributed among plants and animals. Most
of them have agglutinating activity towards vertebrate erythrocytes and other
animal cells, due to the presence of multiple carbohydrate recognition domains
which bind to cell surface sugars. A great number of invertebrate lectins have
been described in the last two decades: they show different specificities,
sizes and physico-chemical properties and are believed to be involved in
various processes, such as cell-cell interaction, fertilisation, morphogenesis and defence reactions.
Ascidians are
invertebrate chordates phylogenetically close to
vertebrates and the study of their immune responses can contribute to a better
understanding of the complex immune system of vertebrates.
In compound ascidians, lectins play an
important role in opsonisation of foreign particles or cells having entered the
organism. They can also induce cell proliferation and enhance the recruitment
of immunocytes to the infection area. In the compound ascidian Botryllus schlosseri, our model
organism, we recently identified a rhamnose-binding lectin (BsRBL) which can
recruit phagocytes, activate their respiratory burst with the consequent
production of microbicidal reactive-oxygen species, and stimulate phagocytosis
of foreign target cell by opsonising them and inducing cytoskeletal changes in
phagocytes. In addition, BsRBL induces the synthesis and release, by cytotoxic
morula cells, of cytokines recognised by anti-IL-I and anti-TNF antibodies,
with chemotactic activity towards cytotoxic immunocytes. It also triggers the
degranulation of morula cells with the consequent release of the cytotoxic
enzyme phenoloxidase. Results suggest an important role of BsRBL in Botryl/us
immunobiology and support the existence of a cross-talk between B. schlosseri immunocytes.
3. The 9th Intl. Vanadium Symposium, June 29th - July 2nd, 2014, Univ. of
Padova, Padova, Italy
Vanadium Accumulation in Ascidians: An
Overview as a System. T.
Ueki, N. Yamaguchi, Romaidi, Y. Isagob, H. Tanahashi. ueki@hiroshima-u.ac.jp
Ascidians are well known to accumulate
extremely high levels of vanadium in their blood cells. The concentration of
vanadium is determined in each species, and the highest one reaches 350 mM, which
corresponds to 107 times that of sea water. How and why ascidians accumulate
vanadium at such an extremely high levels? To address these questions, our
research group has been trying to identify genes and proteins responsible for
the accumulation and reduction of vanadium in vanadocytes, one type of blood
cells, as well as the process of vanadium transport from sea water to blood
cells through the branchial sac, intestine and blood plasma. Here, we would
like to overview the accumulation steps as a system, especially related to
concentration and chemical species of vanadium at each step, as were
experimentally determined in a vanadium-rich ascidian Ascidia sydneiensis
samea. Comprehensive analysis on each organ has already revealed several
categories of protein families, such as vanadium-binding proteins and vanadium
transporters. We would like to discuss possible participation of proteins at
each step from biochemical viewpoint.
4. The 5th Int.l
Conference on Green Technology, November 7th - 8th, 2014, National Islamic
University, Malang, Indonesia.
Biotechnology and biomimetics: lessons
from marine animals.
T. Ueki,
Dept. of Biol., Graduate School of Sci., Hiroshima Univ. ueki@hiroshima-u.ac.jp
Marine animals are regarded as one of the
best sources of materials for use in biotechnological or biomimetic
applications. Our research group has attempted to identify metal-related genes
from marine animals and to apply them in selective metal accumulation or efficient
metal-removal systems. The most prominent factor is a family of
vanadium-binding proteins, Vanabins, which were found only in vanadium-rich
ascidians and were used successfully in copper and vanadium accumulation
systems. In addition, we recently began to investigate the attachment and
anti-fouling mechanisms of ascidians. In this paper, we review the history of
biotechnology and biomimetics regarding marine animals, especially as they
relate to our investigations of ascidians. We also introduce the situation in
Japan and summarize biotechnological and biomimetic applications and provide a
future prospective.
5. 60th Congresso de Genetica 2014, Guarujá SP, Brazil, August 26-29, 2014
Regeneration in ascidians:
implications of developmental synchrony and ncRNAs.
Arianna S.
Gutierrez1, Cristian A. Velandia2, Clara Bermudez-Santana2, Arjan
Gittenberger3,4, Federico D. Brown1,5
1Department
of Biological Sciences, Universidad de los Andes, Bogotá, Colombia, fd.brown@usp.br
2Department
of Biology, Universidad Nacional de Colombia, Bogotá, Colombia, cibermudezs@unal.edu.co
3GiMaRIS,
Leiden, The Netherlands, Gittenberger@gimaris.com
4Naturalis
Biodiversity Center and Institute of Biology, Leiden University, The Netherlands
5Department
of Zoology, Instituto de Biociencias, Univ. de Sao Paulo, Brazil fdbrown@usp.br
To understand how major reproductive modes
evolve during life history transitions and to explore ncRNA involvement in
these transitions, we experimentally disrupted asexual modules of development
of a colonial ascidian Symplegma
brakenhielmi, and compared the genomes of solitary and colonial tunicates
to associate specific ncRNA to putative mechanisms of asexual reproduction,
regeneration, and budding. In the Styelidae, basal species are solitary or
social (i.e. individuals or aggregates), whereas derived species are colonial
(i.e. integrated individuals within a common tunic). Basal colonial styelid S. brakenhielmi does not synchronize
budding and its buds generally develop independently at extracorporeal vessels
that connect the individuals of the colony, whereas derived styelids Botryllus spp. and Botrylloides spp. bud all forming individuals synchronously, and
generally develop by evagination of the lateral epidermis of adults. To show
that S. brakenhielmi individuals show
complete independence in development, we carried systemic bud or zooid removal
in the colony and compared our results to previous observations in Botryllus
schlosseri. Next, we studied hemocytes and analyzed proliferation in S. brakenhielmi to identify putative
circulatory progenitor cells. Budding in ascidians requires a permanent supply
of progenitor cells likely regulated by ncRNA pathways. Therefore by comparing
the genomes of colonial ascidians Didemnum
vexillum and Botryllus schlosseri
to the genomes of solitary tunicates Ciona
spp., Molgula spp., and Oikopleura dioica we generate ncRNA
predictions and attempt to associate specific loci to the evolution of asexual
modes of reproduction and regeneration. Our results support a stepwise
integration of budding synchrony and developmental interaction of individuals
during the evolution of coloniality, and raises new questions about ncRNA
regulation in stem cell function of colonial marine chordates.
6. TWAS 25th General Meeting, Muscat, Oman, October 26-29, 2014
[TWAS is the hub for a global network of scientists and organizations working
to advance science in the developing world]
Modular development and evolution of
clonal marine chordates
A. S.
Gutierrez1, C. A. Velandia2, C. Bermudez-Santana2, A. Gittenberger3, F. D.
Brown1,4
1Departamento
de Ciencias Biologicas, Universidad de los Andes, Bogota, Colombia
2Departamento
de BiologÃa, Universidad Nacional de Colombia, Bogota, Colombia
3GiMaRIS,
Leiden, The Netherlands
4Departamento
de Zoologia, Instituto de Biociencias, Universidade de Sao Paulo, Brazil
Colonial styelid ascidians form two types of
organization. In derived species (i.e. botryllid ascidians) individuals of the
colony are connected and integrated within a common tunic and new individuals
form in weekly cycles of budding; in contrast, other colonial styelids present
an aggregate organization, in which individuals are embedded within their own
tunic and remain connected by extracorporeal tissues. The latter develop
independently and by asynchronous budding. A sister species of the botryllids Symplegma brakenhielmi presents an
intermediate form, i.e. individuals are well integrated, but development occurs
in an apparently independent manner. To understand how major reproductive modes
evolve during life history transitions, we experimentally disrupted asexual
modules of development of a colonial ascidian Symplegma brakenhielmi by systemic bud or zooid removals in the
colony and identification of putative circulatory progenitor cells involved in
asexual reproduction. Budding in ascidians requires a permanent supply of
progenitor cells likely regulated by ncRNA pathways. To associate specific
ncRNAs to putative mechanisms of asexual reproduction, we compared the genomes
of solitary Ciona intestinalis and C. savignyi and colonial Didemnum vexillum and Botryllus schlosseri. We generated ncRNA
predictions and attempted to associate specific loci to the evolution of
asexual modes of reproduction and regeneration. Our results support a stepwise
integration of budding synchrony and developmental interaction of individuals
during the evolution of coloniality, and raise new questions about ncRNA
regulation in stem cell function of colonial marine chordates.
7. Intl. Invasive Sea Squirt
Conference 5
(IISSC5) Woods Hole, Oct. 29-31, 2014. Many thanks to Mary Carman for
organizing this very successful meeting. A few contributed abstracts are
included below, but for a pdf of the complete program including all the
abstracts, go to the bottom of the conference website page.
http://www.whoi.edu/main/sea-squirt-conference-v
Or
the direct link for the full program and abstracts is http://www.whoi.edu/fileserver.do?id=199184&pt=2&p=187649
The highlight of this year’s program was
Walter Rhee’s Korean stir fry with Styela
clava, plus raw Halocynthia roretzi
(from Japan, imported frozen out of tunic) to dip in Korean hot sauce.
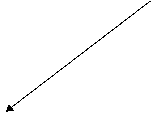



Left: Walter Rhee (Univ.
of Hawaii Dept. of Food Sci.); middle: closeup; arrow marks whole small Styela clava in tunic; right: Halocynthia roretzi thawed.
a) Wild and cultured edible tunicates:
a review. Richard Karney1, Walter
Rhee2, Gretchen Lambert3, Mary R. Carman4
1Martha’s Vineyard Shellfish Group,
Inc. Oak Bluffs, MA 02557
2University of Hawaii, Food Science
& Human Nutrition Dept., Honolulu, HI 96822
3University of Washington Friday
Harbor Laboratories, Friday Harbor, WA 98250
4Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA 02543
Most tunicate species are not edible by humans but some solitary
stolidobranchs in the Styelidae and Pyuridae families are harvested wild or
cultured for food. The main species are Halocynthia aurantium, H. roretzi,
Microcosmus hartmeyeri, M. sabatieri, M. sulcatus, M. vulgaris, Polycarpa
pomaria, Pyura chilensis, Styela clava, and S. plicata, and they may
be eaten raw, cooked, dried or pickled. Historically the Maoris ate Pyura
pachydermatina in New Zealand and aboriginal people ate P. praeputialis in
Australia, though it is now only used for fishing bait. There is a large market
for cultured tunicates, especially among Asian populations. S. clava and
S. plicata have become extremely abundant in many countries as non-native
introductions; they could easily be harvested and sold as seafood, as could
common species that have not previously been consumed such as Herdmania
pallida. Disease and overexploitation can reduce cultured product and wild
populations. Recently, the disease ‘soft tunic syndrome’ caused up to a 70% loss
of H. roretzi crop in Korea, while harvesting wild P. chilensis reduced
their richness three fold in some parts of Chile. Most aquaculture operations
are located in bays with urban runoff where pollutants including heavy metals
and toxic substances could accumulate in tunicates. Natural disasters like
tsunamis will also negatively impact aquaculture. Nevertheless, with proper
controls and monitoring, certain edible tunicate species that are currently an
underutilized food in many parts of the world could be easily cultivated or the
huge numbers of invaders could be harvested and marketed.
b) Alternative menthol
sources for ascidian relaxation. Lauren M. Stefaniak1 and Johann
Heupel2
1James H. Oliver, Jr.
Institute for Coastal Plain Science, Georgia Southern University, Statesboro,
GA lstefaniak@georgiasouthern.edu
2Marine Science Magnet High
School of Southeastern Connecticut, Groton, CT
Proper preservation of ascidians, particularly
colonial ascidians, for morphological taxonomy requires specimens to be relaxed
before fixation. Menthol crystals, either floated in
sea water or dissolved in ethanol, are highly effective at relaxing ascidians,
but are not readily obtainable at short notice. We compared the relaxation
efficacy and cost of three sources of concentrated menthol (100% peppermint
oil, peppermint extract, and Altoids® mints) that are readily available in
stores to menthol crystals and menthol dissolved in ethanol. All menthol
sources tested successfully relaxed Ciona intestinalis individuals in
that the ascidians no longer reacted to a glass probe inserted in the oral
siphon. However, full extension of specimens was more common when menthol
crystals or menthol-in-ethanol were used. Being able to dry and reuse menthol
crystals also make them a relatively low cost menthol source. Therefore,
peppermint oil, peppermint extract, and Altoids® mints are all useful
substitutes for menthol crystals, but menthol crystals, when available, remain
the first choice when relaxing ascidians for preservation.
c) A
potential induced physical defense in a didemnid ascidian. Lauren M.
Stefaniak1, Richard W. Osman2, and Robert B. Whitlatch3
1James H. Oliver, Jr. Institute
for Coastal Plain Science, Georgia Southern University, Statesboro, GA lstefaniak@georgiasouthern.edu
2Smithsonian Environmental
Research Center, Edgewater, MD
3Department of Marine
Sciences, University of Connecticut, Groton, CT
Spicules in ascidians are thought to serve a
variety of functions including structural support, shading of photosynthetic
symbionts, and defense. Like many species of didemnid ascidians, Didemnum
vexillum Kott, 2002 has stellate, calcium carbonate spicules embedded in
its tunic. In D. vexillum, spicule concentration is highly variable even
within colonies, however, the mechanisms controlling spicule density are not
well known. Qualitative observations showed that partial predation by dove
snails (Costanachis spp.) on juvenile D. vexillum colonies
resulted in whiter (greater spicule concentration) colonies, suggesting the
potential for an induced physical defense. The inducible nature of spicules as
a defense was tested by exposing
juvenile D. vexillum colonies to dove snail predation, potential dove
snail chemical cues, or abiotic physical damage from a razor blade. The norm of
reaction to increasing degrees of physical damage was tested by exposing
colonies to different amounts of abiotic physical damage. We found that partial
predation by dove snails and abiotic physical damage result in increased
spicule density, while the presence of a snail without a predation event did
not. Increasing the degree of physical damage to a colony resulted in an
increased concentration of spicules.
d) Harbor
networks as introduction patchworks: Contrasting distribution patterns of
native and introduced ascidians.
López-Legentil
S1, Legentil ML, Erwin PM1, Turon X2 lopezlegentils@uncw.edu
1Dept. of Biology & Marine Biology,
Univ. of North Carolina, Wilmington NC
2Center for Advanced Studies of Blanes,
Spain
Harbors and marinas are well known
gateways for species introductions in marine environments but little work has
been done to ascertain relationships between species diversity, harbor type,
and geographic distance. Here, we sampled ascidians from 32 harbors along ca.
300 km of the NW Mediterranean coast and investigated patterns of distribution
and spread related to harbor type (recreational, fishing, commercial) and
geographic location using multivariate techniques. In total, 28 ascidians were
identified at the species level and another 9 at the genus level based on
morphology and genetic barcoding. Eight species were assigned to introduced
forms, 15 were given native status and 5 were classified as cryptogenic. Aplidium accarense was reported for the
first time in the Mediterranean and was especially abundant in 23 of the harbors.
Introduced and cryptogenic species were abundant in most of the surveyed
harbors, while native forms were rare and restricted to a few harbors.
Significant differences in the distribution of ascidians according to harbor
type and latitudinal position were observed. These differences were exclusively
due to the distribution of introduced species. We obtained a significant
correlation between geographic distance and ascidian composition, indicating
that closely located harbors shared more ascidian species among them. This
study showed that harbors act as dispersal strongholds for introduced species,
with native species only appearing sporadically, and that harbor type and
geographic location should also be considered when developing management plans
to constrain the spread of non-indigenous species in highly urbanized
coastlines.
e) Ascidian rapid assessment survey of
North Carolina and Georgia marinas July 2014.
1Susanna López-Legentil, 2Gretchen
Lambert, and 3Lauren M. Stefaniak
1Department of Biology & Marine
Biology, Center for Marine Science, University of North Carolina Wilmington,
5600 Marvin K. Moss Ln, Wilmington NC 28409; lopezlegentils@uncw.edu
2University of Washington Friday Harbor
Labs, 620 Univ. Road, Friday Harbor WA 98250; gretchen.lambert00@gmail.com
3James H. Oliver, Jr., Institute for
Coastal Plain Science, Georgia Southern University, P.O.Box 8056, Statesboro,
Georgia 30460; lstefaniak@georgiasouthern.edu
We sampled 17 marinas along the North
Carolina coast from July 16-23, 2014, from Southport to sites on Cape Hatteras,
for native and introduced ascidians. Five sites had no ascidians, presumably
due to low salinity resulting from prolonged and unusually heavy rainfall
during the previous months. In fact, when compared with results obtained for
Wrightsville Beach after a quick survey the previous month (June 2014), some
species had totally disappeared, notably Didemnum
duplicatum, which was extremely abundant in June. Data will be presented on
all sites, the species present and their abundance. A total of 19 species were
collected and barcoded. Of the 12 sites with ascidians, the most widespread and
abundant species was the non-native Styela
plicata (11 sites). Ascidia
interrupta, Perophora viridis and
Polyandrocarpa aff. maxima were present at 6 sites; the former 2
are considered native, the latter cryptogenic. We thank Stephanie Villalobos for help with collecting.
One of us (L.M.S.) sampled ascidians at 15
public boat launches along the Georgia coast and the Grays Reef National Marine
Sanctuary dock from July 28-August 1. Surprisingly, only 4 species were recorded,
with Molgula manhattensis the most
widespread while Styela plicata was
recorded at only one site. Low ascidian species richness may be due to pulses
of low salinity during low tide at the sites sampled. We thank Brianne Varnerin for help with
collecting in Georgia.
f) Marine bioinvasions in anthropogenic and natural habitats: an investigation of nonindigenous ascidians in British Columbia. Christina Simkanin1,2*, John Dower2, Glen Jamieson3, Thomas Therriault3
1 Smithsonian Environmental Research
Center, Edgewater, MD 21037, USA
2 University of Victoria, Victoria,
B.C. V8W 3N5, Canada
3 Department
of Fisheries and Oceans, Nanaimo, B.C. V9T 6N7, Canada
As part of my doctoral research, I examined
patterns of marine invasions across anthropogenic and natural habitats and
explored processes that influence the establishment and spread of ascidian
invaders. The goals of my work were four-fold. First, I examined the habitat
distribution of marine nonindigenous species (NIS) spanning several taxonomic
groups and geographical regions. Second,
I conducted subtidal surveys in anthropogenic and natural habitats and
investigated the distribution of nonindigenous ascidians on Southern Vancouver
Island, British Columbia, Canada. Third, I tested methods for in-situ larval inoculations and utilized
these techniques to manipulate propagule supply and assess post-settlement
mortality of ascidians across habitat types. And fourth, I investigated the
role of biotic resistance, through predation by native species, on the survival
of ascidian colonies in anthropogenic and natural habitats. Results showed that anthropogenic habitats
are hubs for marine invasions and may provide beachheads for the infiltration
of nearby natural sites. Field manipulations using the ascidian Botrylloides violaceus as a model
organism, indicated that post-settlement mortality is high and that large
numbers of larvae or frequent introduction events may be needed for successful
initial invasion and subsequent infiltration of natural habitats. Experiments also showed that predation by
native species can limit the survival of B.
violaceus in anthropogenic and natural habitats. These data contribute
knowledge about the patterns and processes associated with habitat
invisibility; provide insight into factors affecting colonization; and supply
valuable information for predicting and managing invasions.
8. 2014
Pyeongchang Convention on Biological Diversity, Pyeongchang, Republic of Korea 29 September-17 October, 2014. Side-event, Improvement of
biodiversity for sustainable development -Strategies and case studies.
Population study of solitary ascidian Herdmania momus
in Jeju Island, Korea.
Changho Yi1,
Ko young wook1, Choi Dong mun1,
Rae-seon Kang1 and Jeong Ha Kim2
1Marine Ecosystem Research Division,
Korea Instit. of Ocean Science & Technology; 2Dept.
of Natural Sci., Sungkyunkwan Univ., Korea
yichangho@kiost.ac
The density and habitat distribution of Herdmania momus
population expanded surprisingly in a few years after their first appearance.
This species is considered as the most dominant benthos in the coast of Jeju Island in recent years. In our study, we
confirmed that water temperature is the main key of population dynamics of H. momus by
controlling mortality and reproductive periods. They represented several
similar characteristics with other invasive ascidians and accordance of
blooming season with other problematic invasive species.
Addendum: In recent additional observations
(not included in this poster), we found their distribution is still extending
although their density has declined. Now we are planning to collect foreign
samples for molecular works, and more communications might be needed. Contact
Changho Yi for a copy of the complete poster.
THESIS ABSTRACTS
1. DETERMINACION MORFOLOGICA Y
MOLECULAR DE ASCIDIAS PROVENIENTES DE SANTA MARTA Y CARTAGENA COLOMBIA. Maria
Jose Paucar, Engineering thesis (June 2014), ESPE, Universidad de las Fuerzas
Armadas, Ecuador, Advisor: Federico D. Brown (Universidad de los Andes &
Universidade de São Paulo)
fdbrown@usp.br
In this investigation the morphological and
molecular identification of 11 ascidians collected in Santa Marta and
Cartagena, Colombia was performed. Morphological species identification was
performed based on literature. This research reports nine (Ascidian species) in
Colombia, out of them, eight have not been identified before whereas one has already
been reported in previous works. Two of these species recently characterized
are invasive and therefore constitute a threat to the habitat of the Caribbean
natural fauna. At the molecular level, the first 7 sequences of mitochondrial
cytochrome oxidase I gene of ascidians to Colombia was obtained. The sequences
were aligned with 30 sequences from GenBank. Phylogenetic reconstruction
methods of NJ, ME, UPGMA and ML were performed in Mega6. This investigation
rebuilt the phylogeny of three of the major groups of tunicates: Polyclinidae,
Styelidae and Pyuridae. In general, although the Styelidae and Polyclinidae
topology has already been reported, Pyuridae showed a topology different from
literature. Cytochrome oxidase I gene presents a low resolution to solve the
phylogeny of tunicates, however it was useful to match
the molecular and morphological results.
NEW PUBLICATIONS
Aguirre,
J. D., Blows, M. W. and Marshall, D. J. 2014. The genetic
covariance between life cycle stages separated by metamorphosis. Proc.
Roy. Soc. B: Biol. Sci. 281: epub
Arienzo,
M., Toscano, F., Di Fraia, M., Caputi, L., Sordino, P., Guida, M., Aliberti, F.
and Ferrara, L. 2014.
An assessment of contamination of the Fusaro Lagoon (Campania
Province, southern Italy) by trace metals. Envir.
Monitoring and Assessment 186: 5731-5747.
Astudillo,
J.-C., Wong, J. C. Y., Dumont, C. P., Bonebrake, T. C. and Leung, K. M. Y.
2014. Status of six non-native marine species in the coastal
environment of Hong Kong, 30 years after their first record.
Bioinvasions Records 3: 123-137.
Auker,
L. A., Majkut, A. L. and Harris, L. G. 2014. Exploring biotic
impacts from Carcinus maenas predation and Didemnum vexillum
epibiosis on Mytilus edulis in the Gulf of Maine. Northeastern
Naturalist 21: 479–494.
Bertanha,
C. S., Januario, A. H., Alvarenga, T. A. and al., e. 2014. Quinone
and hydroquinone metabolites from the ascidians of the genus Aplidium.
Mar. Drugs 12: 3608-3633.
Bezzaouia,
A., Gallo, A., Silvestre, F., Tekaya, S. and Tosti, E. 2014. Distribution
pattern and activity of mitochondria during oocyte growth and maturation in the
ascidian Styela plicata. Zygote 22: 462-469.
Blasiak,
L. C., Zinder, S. H., Buckley, D. H. and Hill, R. T. 2013. Bacterial diversity associated with
the tunic of the model chordate Ciona intestinalis. ISME J. 8:
309-320.
Bouzon,
J. L., Vargas, S. M., Oliveira Neto, J. F., Stoco, P. H. and Brandini, F. P.
2014. Cryptic
species and genetic structure in Didemnum granulatum Tokioka, 1954
(Tunicata: Ascidiacea) from the southern Brazilian coast. Braz. J. Biol. epub:
1-10.
Breton,
G. 2014. Alien or invasive species in the ports of Le Havre,
Antifer and Rouen (Normandy, France). Hydroécologie Appliquée epub:
1-43.
Bridgwood,
S. D., Muñoz, J. and McDonald, J. J. 2014. Catch me if you can! The story of a
colonial ascidian’s takeover bid in Western Australia. Bioinvasions
Records 3: 217-223.
Cannon,
J. T., Kocot, K. M., Waits, D. S., Weese, D. A.,
Swalla, B. J., Santos, S. R. and Halanych, K. M. 2014. Phylogenomic
resolution of the hemichordate and echinoderm clade. Curr.
Biol. epub: 1-19.
Carrion-Cortez,
J., Canales-Cerro, C., Arauz, R. and Riosmena-Rodriguez, R. 2013. Habitat use and
diet of juvenile eastern Pacific hawksbill turtles (Eretmochelys imbricata)
in the North Pacific coast of Costa Rica. Chelonian Conservation and
Biology 12: 235–245.
Castellano,
I., Ercolesi, E. and Palumbo, A. 2014. Nitric oxide affects ERK signaling through
down-regulation of MAP kinase phosphatase levels during larval development of
the ascidian Ciona intestinalis. PLoS One 9: e102907.
Chan,
S. T. S., Henrich, C. J., O'Keefe, B. R., McKee, T. C., McMahon, J. B. and
Gustafson, K. R. 2014. Isolation and identification of
biologically active natural products from marine ascidians. Planta Med. 80:
767-777.
Chen,
W. C., Pauls, S., Bacha, J., Elgar, G., Loose, M. and Shimeld, S. M. 2014. Dissection of a Ciona
regulatory element reveals complexity of cross-species enhancer activity. Dev.
Biol. 390: 261-272.
Cinar,
M. E. 2014. Checklist of the phyla Platyhelminthes, Xenacoelomorpha, Nematoda,
Acanthocephala, Myxozoa, Tardigrada, Cephalorhyncha, Nemertea, Echiura,
Brachiopoda, Phoronida, Chaetognatha, and Chordata (Tunicata, Cephalochordata,
and Hemichordata) from the coasts of Turkey. Turk. J.
Zool. 38: 698-722.
Cooper,
E. L. and Albert, R. 2013.
Tunicates: A vertebrate ancestral source of antitumor compounds. In: Kim, S.-K.
(ed.), Handbook of Anti-cancer Drugs from Marine Origin. pp.
Cooper,
E. L. and Yao, D. 2012.
Diving for drugs: tunicate anticancer compounds. Drug
Discovery Today 17: 636-648.
Coppari,
M., Gori, A. and Rossi, S. 2014.
Size,
spatial, and bathymetrical distribution of the ascidian Halocynthia
papillosa in Mediterranean coastal bottoms: benthic-pelagic coupling
implications. Mar. Biol. 161: 2079-2095.
Cordero,
M., Borbón, H., Román, F. R., Morell, L., Viquez, R., Villegas, L. R., Soto, R.
and Vega, I. 2011.
Chemical and functional characterization of antimicrobial metabolites isolated
from ascidian Rhopalaea birkelandi. Rev. Mar. Cost. 3: 111-125.
Costache,
V., McDougall, A. and Dumollard, D. 2014. Cell cycle arrest and activation of
development in marine invertebrate deuterostomes. Biochem.
Biophys. Res. Comm. 450: 1175–1181.
Crocetta,
F., Marino, R., Cirino, P., Macina, A., Staiano, L., Esposito, R., Pezzotti,
R., Racioppi, C., Toscano, F., De Felice, E., Locascio, A., Ristoratore, F.,
Spagnuolo, A., Zanetti, L., Branno, M. and Sordino, P. 2014. Mutation studies
in ascidians: A review. Genesis epub:
Diniz,
G. S., Barbarino, E., Oiano-Neto, J., Pacheco, S. and Lourenco, S. O. 2014. Proximate
composition of marine invertebrates from tropical coastal waters, with emphasis
on the relationship between nitrogen and protein contents. Latin Amer. J. Aquat. Res. 42: 332-352.
Dishaw,
L. J., Cannon, J. P., Litman, G. W. and Parker, W. H. 2014. Immune-directed support of rich
microbial communities in the gut has ancient roots. Dev. Comp. Immunol. 47:
36-51.
Edwards,
K. F. and Stachowicz, J. J. 2011.
Spatially stochastic settlement and the coexistence of
benthic marine animals. Ecology 92: 1094-1103.
Espositoa,
R., Racioppia, C., Pezzotti, M. R., Branno, M., Locasciob, A., Ristoratoreb, F.
and Spagnuolob, A. 2014.
The ascidian pigmented sensory organs: structures and developmental programs.
Genesis epub:
Franchi
, N. and Ballarin,
L. 2014. Preliminary characterization of complement in a colonial tunicate: C3,
Bf and inhibition of C3 opsonic activity by compstatin. Dev. Comp. Immunol. 46:
430–438.
Franchi
, N., Hirose, E.
and Ballarin, L. 2014. Cellular aspects of allorecognition in
the compound ascidian Botrylloides simodensis. ISJ 11:
219-223.
Gallego,
V., Perez, L., Asturiano, J. F. and Yoshida, M. 2014. Sperm motility parameters and
spermatozoa morphometric characterization in marine species: a study of swimmer
and sessile species. Theriogenology 82: 668-676.
Gasparini,
F., Caicci, F., Rigon, F., Zaniolo, G. and Manni, L. 2014. Testing an unusual
in vivo vessel network model: a method to study angiogenesis in the colonial
tunicate Botryllus schlosseri. Sci. Reports epub: 1-11.
Gasparini,
F., Manni, L., Cima, F., Zaniolo, G., Burighel, P., Caicci, F., Franchi , N., Schiavon, F., Rigon, F., Campagna, D. and
Ballarin, L. 2014. Sexual and asexual reproduction in the
colonial ascidian Botryllus schlosseri. Genesis epub:
1-16.
Haupaix,
N., Abitua, P. B., Sirour, C., Yasuo, H., Levine, M. and Hudson, C. 2014. Ephrin-mediated restriction of ERK1/2
activity delimits the number of pigment cells in the Ciona CNS. Dev.
Biol. 394: 170-180.
Hirose,
E., Iskandar, B. H. and Wardiatno, Y. 2014. Photosymbiotic
ascidians from Pari Island (Thousand Islands, Indonesia). Zookeys 422:
1–10.
Hirose,
E., Kumagai, A., Nawata, A. and Kitamura, S.-I. 2014. Azumiobodo hoyamushi,
the kinetoplastid causing soft tunic syndrome in ascidians, may invade through
the siphon wall. Diseases of Aquat. Org. 109:
251–256.
Hong,
S. H., Kwone, J. T., Lee, J. H., Lee, S., Lee, A. Y., Cho, W. Y., Bat-Erdene,
M., Choi, B. D. and Cho, M. H. 2014. Ascidian tunicate extracts attenuate rheumatoid arthritis
in a collagen-induced murine model. Nat. Prod. Commun.
9: 847-851.
Ibrahim,
S. R., Mohamed, G. A., Shaala, L. A., Youssef, D. T. and Gab-Alla, A. A. 2014. Didemnacerides A and B: two new
glycerides from Red Sea ascidian Didemnum species. Nat. Prod. Res. 28:
1591-1597.
Iitsuka,
T., Mita, K., Hozumi, A., Hamada, M., Satoh, N. and Sasakura, Y. 2014. Transposon-mediated
targeted and specific knockdown of maternally expressed transcripts in the
ascidian Ciona intestinalis. Sci. Reports 4:
Ilut,
D., Nydam, M. L. and Hare, M. P. 2014. Defining loci in restriction-based
reduced representation genomic data from nonmodel species: Sources of bias and
diagnostics for optimal clustering. BioMed.
Research Intl. epub:
Jeffery,
W. R. 2014. The tunicate Ciona: a model system for understanding the
relationship between regeneration and aging. Invert. Repro. & Dev. epub: 1-6.
Jeffery,
W. R. 2014. Closing the wounds: one hundred and twenty five
years of regenerative biology in the ascidian Ciona intestinalis.
Genesis epub: 1-18.
Jeffery,
W. R. 2014. Distal regeneration involves the age dependent activity of
branchial sac stem cells in the ascidian Ciona intestinalis.
Regeneration epub: 1-18.
Kamiya,
C., Ohta, N., Ogura, Y., Yoshida, K., Horie, T., Kusakabe, T. G., Satake, H.
and Sasakura, Y. 2014.
Nonreproductive role of gonadotropin-releasing hormone in the
control of ascidian metamorphosis. Dev. Dyn. 243: 1524-1535.
Karaiskou,
A., Swalla, B. J., Sasakura, Y. and J.-P., C. 2014. Metamorphosis in
solitary ascidians. Genesis special issue, in press: 1-14.
Karlson,
R. H. and Osman, R. W. 2012.
Species composition and geographic distribution of invertebrates in fouling
communities along the east coast of the USA: a regional perspective. Mar. Ecol.
Prog. Ser. 458: 255–268.
Kawaguchi,
A., Utsumi, N., Morita, M., Ohya, A. and Wada, S. 2014. Application
of the cis-regulatory region of a heat-shock protein 70 gene to heat-inducible
gene expression in the ascidian Ciona intestinalis. Genesis epub:
Kelmo,
F., Bell, J. J., Moraes, S. S., Tourinho Gomes, R., Mariano-Neto, E. and
Attrill, M. J. 2014.
Differential responses of emergent intertidal coral reef fauna to a large-scale
El-Nino Southern Oscillation event: sponge and coral resilience. PLoS One 9:
e93209.
Kim, H.
J., Park, J. S., Park, K. H., Shin, Y. K. and Park, K. I. 2014. The
kinetoplastid parasite Azumiobodo hoyamushi, the causative agent of soft
tunic syndrome of the sea squirt Halocynthia roretzi, resides in the
East Sea of Korea. J. Invert. Pathol. 116: 36-42.
Kim,
Y. O., Park, S., Nam, B. H., Jung, Y. T., Kim, D. G., Bae, K. S. and Yoon, J.
H. 2014.
Description of Lutimonas halocynthiae sp. nov.,
isolated from a golden sea squirt (Halocynthia aurantium),
reclassification of Aestuariicola saemankumensis as Lutimonas
saemankumensis comb. nov. and
emended description of the genus Lutimonas. Intl. J.
Syst. & Evol. Microbiol. 64:
1984-1990.
Kim,
Y. O., Park, S., Nam, B. H., Park, J. M., Kim, D. G. and Yoon, J. H. 2014. Litoreibacter ascidiaceicola
sp. nov., isolated from the golden sea squirt Halocynthia
aurantium. Intl. J. Syst. & Evol. Microbiol. 64: 2545-2550.
Koplovitz,
G., Hirose, E., Hirose, M. and Shenkar, N. 2014. Being green in the Red Sea - the
photosymbiotic ascidian Diplosoma simile (Ascidiacea: Didemnidae) in the
Gulf of Aqaba. Systematics and Biodiversity epub: 1-9.
Kourakis,
M. J., Reeves, W., Newman-Smith, E., Maury, B., Abdul-Wajid, S. and Smith, W.
C. 2014. A
one-dimensional model of PCP signaling: polarized cell behavior in the
notochord of the ascidian Ciona. Dev. Biol. 395: 120-130.
Langenbacher,
A. D., Rodriguez, D., Di Maio, A. and De Tomaso, A. W. 2014. Whole-mount
fluorescent in situ hybridization staining of the colonial tunicate Botryllus
schlosseri. Genesis epub:
Lavender,
J. T., Dafforn, K. A. and Johnston, E. L. 2014. Meso-predators: A confounding
variable in consumer exclusion studies. J. Exp. Mar. Biol. Ecol. 456:
26-33.
Liu,
G., Liu, M., Wei, J., Huang, H., Zhang, Y., Zhao, J., Xiao, L., Wu, N., Zheng,
L. and Lin, X. 2014.
CS5931, a novel polypeptide in Ciona savignyi, represses angiogenesis
via inhibiting vascular endothelial growth factor (VEGF) and matrix
metalloproteinases (MMPs). Mar. Drugs 12: 1530-1544.
Lowe,
E. K., Swalla, B. J. and Brown, C. T. 2014. Evaluating a
lightweight transcriptome assembly pipeline on two closely related ascidian
species. PeerJ epub: 1-10.
Lu,
Y., Zhuang, Y. and Liu, J. 2014. Mining antimicrobial
peptides from small open reading frames in Ciona intestinalis. J.
Peptide Sci. 20: 25-29.
Luttrell,
S. and Swalla, B. J. 2014.
Genomic and evolutionary insights into chordate origins.
In: Moody, S. (ed.), Principles of Developmental Genetics, 2nd ed. San Diego,
Elsevier Press, pp. 115-128.
Mabrouk,
L., Ben Brahim, M., Hamza, A. and Bradai, M.-N. 2014. Diversity and temporal
fluctuations of epiphytes and sessile invertebrates on the rhizomes of Posidonia
oceanica in a seagrass meadow off Tunisia. Mar. Ecol. 35: 212-220.
Maghsoudlou,
A. and Rahimian, H. 2014. Contribution to the knowledge of cotylean flatworms (Turbellaria,
Polycladida) from Iranian coasts: Introducing a new species, with remarks on
new records. Zootaxa 3860: 325-342.
Manriquez,
P. H., Fica, E., Ortiz, V. and Castilla, J. C. 2014.
Marine bio-encrusting in the Chacao channel, Chile: a study of potential
interactions with man-made structures. Rev. Biol. Mar. y Oceanog. 49:
243-265.
Martí-Solans,
J., Ferrández-Roldán, A., Godoy-Marín, H., Badia-Ramentol, J., Torres-Águila,
N. P., Rodríguez-Marí, A., Bouquet, J. M., Chourrout, D., Thompson, E. M., Albalat,
R. and Cañestro, C. 2014. Oikopleura dioica culturing made easy: A
low-cost facility for an emerging animal model in EvoDevo. Genesis
epub.
McDonald,
J. I., Wilkens, S. L., Stanley, J. A. and Jeffs, A. G. 2014. Vessel generator noise
as a settlement cue for marine biofouling species. Biofouling 30:
741-749.
Mojib,
N., Amad, M., Thimma, M., Aldanondo, N., Kumaran, M. and Irigoien, X. 2014. Carotenoid metabolic profiling and
transcriptome-genome mining reveal functional equivalence among blue-pigmented
copepods and appendicularia. Molec. Ecol. 23:
2740-2756.
Moore,
A. M., Vercaemer, B., DiBacco, C., Sephton, D. and Ma, K. C. K. 2014. Invading Nova
Scotia: first records of Didemnum vexillum Kott, 2002 and four more non-indigenous
invertebrates in 2012 and 2013. Bioinvasions Records 3: 225-234.
Moreno,
T. R., Faria, S. B. and Rocha, R. M. 2014. Biogeography of Atlantic and
Mediterranean ascidians. Mar. Biol. 161: 2023-2033.
Nakamura,
J., Yoshida, K., Sasakura, Y. and Fujiwara, S. 2014. Chondroitin
6-O-sulfotransferases are required for morphogenesis of the notochord in the
ascidian embryo. Dev. Dyn. 243: 1637-1645.
Nakamura,
J., Tetsukawa, A. and Fujiwara, S. 2015. Chondroitin 4-O-sulfotransferases are
required for cell adhesion and morphogenesis in the Ciona intestinalis
embryo. Dev. Growth Differ. in press.
Nall,
C. R., Guerin, A. J. and Cook, E. J. 2014. Rapid assessment of marine non-native
species in northern Scotland and a synthesis of existing Scottish records.
Aquatic Invasions 9: 1-15.
Nishida,
H. and Stach, T. 2014. Cell lineages and fate maps in tunicates: conservation
and modification. Zool. Sci. 31:
645–652.
Nunez-Pons,
L. and Avila, C. 2014.
Defensive metabolites from Antarctic invertebrates: does energetic content
interfere with feeding repellence? Mar. Drugs 12: 3770-3791.
Ohtsuka,
Y., Matsumoto, J., Katsuyama, Y. and Okamura, Y. 2014. Nodal signaling
regulates specification of ascidian peripheral neurons through control of the
BMP signal. Development 141: 3889-3899.
Ooishi,
S. 2014. Botryllophilus symmetricus, new species (Copepoda: Cyclopoida:
Ascidicolidae), living in a compound ascidian (Synoicum) from Madagascar. Proc.
Biol. Soc. Wash. 127: 340-352.
Ooishi,
S. 2014. Botryllophilus kozloffi, new species (Copepoda: Cyclopoida:
Ascidicolidae), living in the compound ascidian Clavelina lepadiformis
(Müller) from Roscoff, France. Proc. Biol. Soc. Wash. 127: 483-495.
Ooishi,
S. 2014. Botryllophilus millari, new species (Copepoda: Cyclopoida:
Ascidicolidae), living in the compound ascidian Eudistoma caeruleum
(Sluiter) from Madagascar. Proc. Biol. Soc. Wash. 127: 496-509.
Oonuma,
K., Hirose, D., Takatori, N. and Saiga, H. 2014. Analysis of
the transcription regulatory mechanism of Otx during the development of the
sensory vesicle in Ciona intestinalis. Zool. Sci. 31:
565-572.
Orton,
J. H. 2013. The ciliary mechanisms on the gill and the mode
of feeding in Amphioxus, ascidians, and Solenomya togata. J. Mar.
Biol. Ass. U.K. 10: 19 - 49.
Page,
M. J., Willis, T. J. and Handley, S. J. 2014. The colonial
ascidian fauna of Fiordland, New Zealand, with a description of two new
species. J. Nat. Hist. epub: 1-37.
Pavao,
M. S. 2015. Ascidian (chordata-tunicata) glycosaminoglycans: extraction,
purification, biochemical, and spectroscopic analysis. Methods
in Molec. Biol. 1229: 79-94.
Perez,
M., Garcia, M., Sanchez, M., Stupak, M., Mazzuca, M., Palermo, J. A. and
Blustein, G. 2014.
Effect of secochiliolide acid isolated from the Patagonian shrub Nardophyllum
bryoides as active component in antifouling paints. Intl. Biodeterioration
& Biodegradation 89: 37-44.
Racioppi,
C., Kamal, A. K., Razy-Krajka, F., Gambardella, G., Zanetti, L., di Bernardo,
D., Sanges, R., Christiaen, L. A. and Ristoratore, F. 2014. Fibroblast growth factor signalling
controls nervous system patterning and pigment cell formation in Ciona
intestinalis. Nature Commun. 5: 4830-.
Razy-Krajka,
F., Lam, K., Wang, W., Stolfi, A., Joly, M., Bonneau, R. and Christiaen, L.
2014.
Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle
specification from primed cardiopharyngeal progenitors. Dev. Cell 29:
263-276.
Reem,
E., Mohanty, I., Katzir, G. and Rinkevich, B. 2013. Population genetic
structure and modes of dispersal for the colonial ascidian Botryllus
schlosseri along the Scandinavian Atlantic coasts. Mar. Ecol. Prog.
Ser. 485: 143–154.
Rimondino,
C., Torre, L., Sahade, R. and Tatián, M. 2014. Sessile
macro-epibiotic community of solitary ascidians, ecosystem engineers in soft
substrates of Potter Cove, Antarctica. Polar Research in press:
Rius,
M., Turon, X., Bernardi, G., Volckaert, F. A. M. and Viard, F. 2014. Marine invasion genetics: from
spatio-temporal patterns to evolutionary outcomes. Biol. Invasions epub:
1-17.
Roure,
A., Lemaire, P. and Darras, S. 2014. An Otx/Nodal regulatory
signature for posterior neural development in ascidians. PLoS Genetics 10:
1-17.
Sasaki,
H., Yoshida, K., Hozumi, A. and Sasakura, Y. 2014. CRISPR/Cas9-mediated
gene knockout in the ascidian Ciona intestinalis. Dev. Growth
& Differ. 56: 499-510.
Sato,
A., Shimeld, S. M. and Bishop, J. D. 2014. Symmetrical
reproductive compatibility of two species in the Ciona intestinalis (Ascidiacea)
species complex, a model for marine genomics and developmental biology.
Zool. Sci. 31: 369-374.
Satoh,
N., Rokhsar, D. and Nishikawa, T. 2014. Chordate evolution
and the three-phylum system. Proc. R. Soc. B: Biol. Sci. 281:
1-10.
Satou,
Y., Hirayama, K., Mita, K., Fujie, M., Chiba, S., Yoshida, R., Endo, T.,
Sasakura, Y., Inaba, K. and Satoh, N. 2014. Sustained
heterozygosity across a self-incompatibility locus in an inbred ascidian.
Molec. Biol. & Evol. epub:
Sawada,
H., Mino, M. and Akasaka, M. 2014.
Sperm proteases and extracellular ubiquitin-proteasome system
involved in fertilization of ascidians and sea urchins. Adv. Exp. Med.
& Biol. 759: 1-11.
Sawada,
H., Morita, M. and Iwano, M. 2014.
Self/non-self recognition mechanisms in sexual reproduction: new insight into
the self-incompatibility system shared by flowering plants and hermaphroditic
animals. Biochem. Biophys.
Res. Comm. 450: 1142-1148.
Seebens,
H., Gastn er, M. T. and Blasius, B. 2013. The risk of marine bioinvasion caused
by global shipping. Ecology Letters 16: 782–790.
Shaala,
L. A., Youssef, D. T., Ibrahim, S. R., Mohamed, G. A., Badr, J. M., Risinger,
A. L. and Mooberry, S. L. 2014. Didemnaketals f and g, new
bioactive spiroketals from a red sea ascidian Didemnum species.
Mar. Drugs 12: 5021-5034.
Shiba,
K. and Inaba, K. 2014. Distinct roles of soluble and
transmembrane adenylyl cyclases in the regulation of flagellar motility in Ciona
sperm. Int. J. Mol. Sci. 15: 13192-13208.
Shibuya,
M., Hatano, M. and Kawamura, K. 2014. Interactive histone acetylation and
methylation in regulating transdifferentiation-related genes during tunicate
budding and regeneration. Dev. Dyn. epub:
Shin,
Y. K., Nam, K. W., Park, K., Yoon, J. M. and Park, K. I. 2014. Quantitative
assessment of Azumiobodo hoyamushi distribution in the tunic of soft
tunic syndrome inverted question markaffected ascidian Halocynthia roretzi
using real-time polymerase chain reaction. Parasites & Vectors 7:
Sievers,
M., Dempster, T., Fitridge, I. and Keough, M. J. 2014. Monitoring biofouling communities
could reduce impacts to mussel aquaculture by allowing synchronisation of
husbandry techniques with peaks in settlement. Biofouling 30: 203-212.
Sokolowski,
A., Szczepanska, A., Richard, P., Kedra, M., Wolowicz, M. and Weslawski, J. M.
2014. Trophic
structure of the macrobenthic community of Hornsund, Spitsbergen, based on the
determination of stable carbon and nitrogen isotopic signatures. Polar Biol. 37:
1247-1260.
Song,
S. H., Kim, J. E., Lee, Y. J., Kwak, M. H., Sung, G. Y., Kwon, S. H., Son, H.
J., Lee, H. S., Jung, Y. J. and Hwang, D. Y. 2014. Cellulose film
regenerated from Styela clava tunics have biodegradability,
toxicity and biocompatibility in the skin of SD rats. J. Materials Sci.
Materials in Med. 25: 1519-1530.
Stefaniak,
L. M. and Whitlatch, R. B. 2014.
Life history attributes of a global invader: factors contributing to the
invasion potential of Didemnum vexillum. Aquat.
Biol. 21: 221–229.
Stolfi,
A., Lowe, E., Racioppi, C., Ristoratore, F., Swalla, B. J., Brown, C. T. and
Christiaen, L. 2014. Divergent mechanisms regulate conserved cardiopharyngeal
development and gene expression in distantly related ascidians. eLife 3: 1-28.
Stolfi,
A., Sasakura, Y., Chalopin, D., Satou, Y., Christiaen, L., Dantec, C., Endo,
T., Naville, M., Nishida, H., Swalla, B. J., Volff, J. N., Voskoboynik, A.,
Dauga, D. and Lemaire, P. 2014. Guidelines for the
nomenclature of genetic elements in tunicate genomes. Genesis
Svanfeldt,
K., Lundqvist, L., Rabinowitz, C., Sköld, H. N. and Rinkevich, B. 2014. Repair of UV-induced DNA damage in shallow
water colonial marine species. J. Exp. Mar. Biol. Ecol. 452: 40–46.
Teske,
P. R., Sandoval-Castillo, J., Waters, J. M. and Beheregaray, L. B. 2014. Can novel genetic analyses help to
identify low-dispersal marine invasive species? Ecol. &
Evol. 4: 2848-2866.
Thompson,
J. M. and Di Gregorio, A. 2014.
Insulin-like genes in ascidians: findings in Ciona and hypotheses on the
evolutionary origins of the pancreas. Genesis epub: 1-23.
Tianero,
M. D., Kwan, J. C., Wyche, T. P., Presson, A. P., Koch, M., Barrows, L. R.,
Bugni, T. S. and Schmidt, E. W. 2014. Species specificity of symbiosis and
secondary metabolism in ascidians. ISME J. epub:
Torre,
L., Abele, D., Lagger, C., Momo, F. and Sahade, R. 2014. When shape matters:
strategies of different Antarctic ascidians morphotypes to deal with
sedimentation. Mar. Env. Res. 99: 179-187.
Tracy,
B. M. and Reyns, N. B. 2014.
Spatial and temporal patterns of native and invasive ascidian
assemblages in a Southern California embayment. Aquatic Invasions 9:
441-455.
Trepos,
R., Cervin, G., Hellio, C., Pavia, H., Stensen, W., Stensvag, K., Svendsen, J.
S., Haug, T. and Svenson, J. 2014.
Antifouling compounds from the sub-arctic ascidian Synoicum pulmonaria:
synoxazolidinones A and C, pulmonarins A and B, and synthetic analogues. J. Nat . Prod. 77: 2105-2113.
Ueki,
T., Uwagaki, M., Yamamoto, S. and Michibata, H. 2014. Participation of
thioredoxin in the V(V)-reduction reaction by
Vanabin2. Biochim. Biophys.
Acta 1840: 3238–3245.
Ueki,
T. and Yamaguchi, N. 2014.
Metal ion metabolism in ascidians: hyperaccumulation of heavy metal from the
sea. Experimental Medicine 32: 123-129.
Ueki,
T., Yamaguchi, N., Isago, Y. and Tanahashi, H. 2014. Vanadium accumulation in ascidians: A
system overview. Coord. Chem. Rev. epub:
Vandepas,
L. E., Lee, S. C., Oliveira, L. M., Hirose, E., Rocha, R. M. and Swalla, B. J.
2014. The native
range of Phallusia nigra: Is it really black and white? Biol. Bull. in press:
Vaugeois,
M., Diaz, F. and Carlotti, F. 2013.
A mechanistic individual-based model of the feeding processes for Oikopleura
dioica. PlOS One 8: e78255.
Veeman,
M. and Reeves, W. 2014.
Quantitative and in toto imaging in ascidians: Working toward an image-centric systems biology of chordate morphogenesis.
Genesis epub:
Vicente,
C. S. and Monniot, F. 2014. The ascidian-associated mysid Corellamysis
eltanina gen.nov., sp.nov. (Mysida, Mysidae,
Heteromysinae): a new symbiotic relationship from the Southern Ocean. Zootaxa 3780:
323-346.
Vizzini,
A., Falco, F. D., Parrinello, D., Sanfratello, M. A., Mazzarella, C.,
Parrinello, N. and Cammarata, M. 2014. Ciona intestinalis interleukin
17-like genes expression is upregulated by LPS challenge. Dev. Comp. Immunol. epub:
Wagner,
E., Stolfi, A., Gi Choi, Y. and Levine, M. 2014. Islet is a key determinant of
ascidian palp morphogenesis. Development 141: 3084-3092.
Won,
T. H., You, M., Lee, S. H., Rho, B. J., Oh, D. C., Oh, K. B. and Shin, J. 2014.
Amino alcohols from the ascidian Pseudodistoma sp. Mar. Drugs 12:
3754-3769.
Wong, G.
W., Zhuo, L., Kimata, K., Lam, B. K., Satoh, N. and Stevens, R. L. 2014. Ancient origin of mast cells. Biochem.
Biophys. Res. Comm. 451: 314-318.
Yakovis,
E. L., Artemieva, A. V. and Fokin, M. V. 2012. Intraspecific variation in
stable isotope signatures indicates no small-scale feeding interference between
a horse mussel and an ascidian. Mar. Ecol. Prog. Ser. 467: 113–120.
Yamada,
A. and Nishida, H. 2014. Control of the number of cell
division rounds in distinct tissues during ascidian embryogenesis. Develop.
Growth Differ. 56: 376–386.
Yokoyama,
T. D., Hotta, K. and Oka, K. 2014. Comprehensive
morphological analysis of individual peripheral neuron dendritic arbors in
ascidian larvae using the photoconvertible protein kaede. Dev. Dyn. 243:
1362-1373.
Yokoyama, T. D., Hotta, K. and Oka, K.
2014. Comprehensive morphological analysis of individual
peripheral neuron dendritic arbors in ascidian larvae using the
photoconvertible protein Kaede. Dev. Dyn. 243: 1362-1373.