|
Trisha Davis
Earl W. Davie/ZymoGenetics Endowed Chair in Biochemistry Professor of Biochemistry
BA 1976, UC Santa Cruz
PhD 1983, Yale University
|
Research
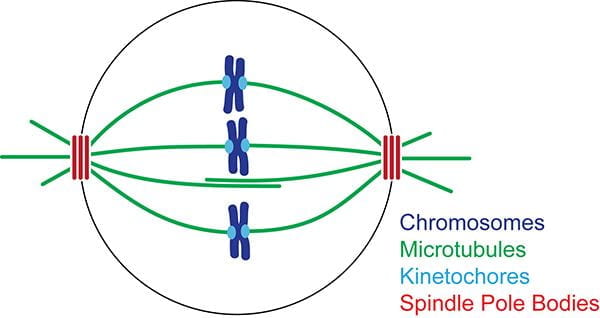
Chromosomes are segregated to daughter cells by a microtubule-based molecular machine, the mitotic spindle. The spindle has two poles, each one carrying an exact complement of chromosomes to each daughter cell. Spindle morphogenesis requires spatially controlled microtubule nucleation. The proteins required for nucleation are known, but they are surprisingly inactive in isolated form. We are studying how these proteins are activated to nucleate microtubules during spindle formation and how their activation is regulated.

Fluorescence microscopy of the S. cerevisiae mitotic spindle. Bipolar spindles are formed by two spindle pole bodies (red), each of which nucleate microtubules (green).
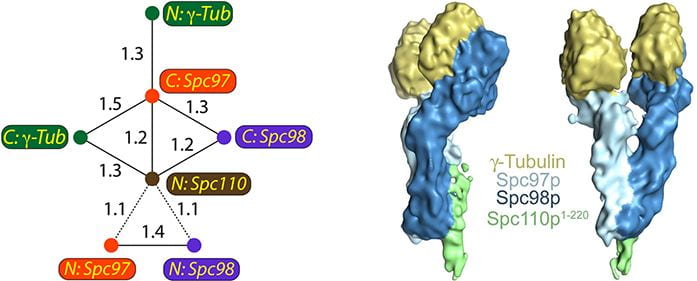
The γ-tubulin small complex (γ-TuSC) associates with the spindle pole body and is required for microtubule nucleation. Left: FRET relationships used to determine the overall architecture of γ-TuSC. Right: structure of a single γ-TuSC.
Chromosomes attach to microtubules via kinetochores, multiprotein organelles that span from the DNA to the microtubule. Once attached to the end of a microtubule, kinetochore components do not turnover; however, the microtubule ends remain dynamic. The addition and removal of thousands of tubulin subunits drive kinetochore oscillations. Persistent attachment to the dynamic microtubule end is essential for accurate chromosome segregation. We are currently investigating how kinetochores maintain their attachments to dynamic microtubules while withstanding tension. We also study the mechanisms that provide for the targeted release of incorrect kinetochore-microtubule attachments.
TIRF microscopy movie of human Ndc80 complex (green) stabilizing a depolymerizing microtubule (red).

Schematic of an optical trapping experiment. A bead decorated with Ndc80 complex can track processively with a dynamic microtubule tip against applied tension.
Publications: