|
James Hurley
Professor of Biochemistry
|
Current Research in the Hurley Lab: Most recently the Hurley lab has turned its attention to understanding how energy metabolism is controlled in photoreceptors. In darkness photoreceptors consume energy rapidly to offset the leakage of ions across the plasma membrane. Light stops that leakage but it introduces qualitatively different energy demands. Dr. Hurley and his colleagues are investigating how the production of energy changes to match the spatial and quantitative changes in energy demand in these neurons.
In past research the Hurley group worked towards developing a molecular understanding of light and dark adaptation in the vertebrate retina. When a retina is dark-adapted an increase in illumination of only a few photons per second can be detected and reported to the brain. When a retina is light adapted it can report changes in illumination on a backgrounds of billions of photons per second. How can a tissue respond with such extreme sensitivity under one condition and avoid saturation under intense stimulation under other conditions? Within the retina there are two types of photoreceptors, rods and cones. Rods are specialized for extraordinary sensitivity; they can detect single photons Cones are specialized for adaptation. Responses of cones do not saturate even under conditions of extremely intense illumination.
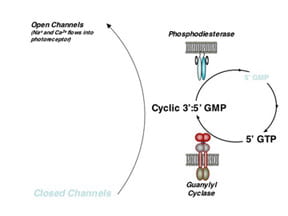
Dr. Hurley and his colleagues focused their efforts on identifying and characterizing proteins in rods and cones that are required for transduction of light into an electrical change in rods and cones. These proteins include rhodopsin, transducin, cGMP phosphodiesterase and guanylyl cyclase. More recently, the group has focused on other enzymes that modulate the phototransduction mechanism, including GCAPs, recoverin and rhodopsin kinase. Measurements of biochemical activities and understanding of regulatory mechanisms have been the focus of those studies.
The focus of the group later broadened to pursue an understanding of how the biochemical activities of these enzymes actually contribute to vision. Animals, both mice and zebrafish, with genetic alterations that affect the activities of specific proteins involved in rod or cone function were analyzed. Sophisticated biochemical, electrophysiological and behavioral strategies were used to directly evaluate the contributions of these proteins to the viability of photoreceptors and to vision over a wide range of illumination conditions.
Publications: