Our research is focused on understanding the basic mechanisms by which blood vessels and myeloid immune cells may drive growth and regeneration of pancreatic islets during development and in diabetes.
Our group is interested in three complementary research areas: 1) Understand the biologic role of bone marrow-derived vasculogenic cells in tissue repair and transplant engraftment; 2) Identify developmental cues provided by myeloid immune cells to pancreatic progenitors and exploit these cues for pancreatic islet cell regeneration; 3) Engineer islet-like tissue microenvironments to enhance transplant engraftment in vivo, and to model diabetogenic insults and therapies ex-vivo.
Understanding the biologic role of bone marrow-derived vasculogenic cells in tissue repair and transplant engraftment.
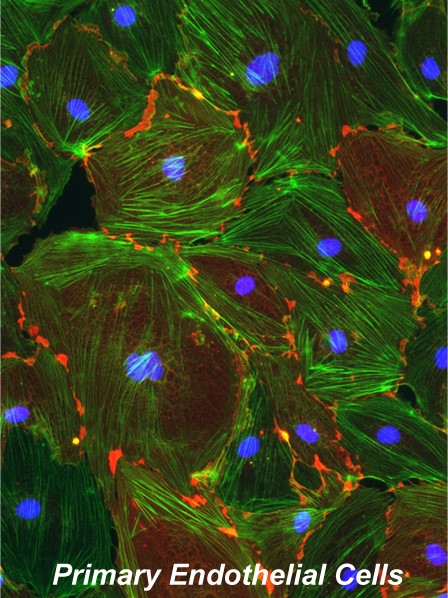 Because of its strategic positioning at the interface between the graft and circulating immune cells, the biological characteristics of the endothelium forming new blood vessels during tissue repair are of primary importance to the survival of transplants. We have identified a subset of human cord blood-derived hemopoietic progenitors that exhibits the developmental potential and functional properties of true endothelial progenitor cells through engagement of angiopoietin-dependent signals. Using animal models, we have further defined unique developmental, phenotypic and functional characteristics of bone-marrow-derived versus tissue-derived endothelial cells in islet transplant settings. Currently, this line of research aims to define the survival/proliferative/ differentiative signals delivered by bone marrow-derived vascular cells to islet tissue, and their involvement in modifying innate immune responses during tissue repair. Understanding these issues may lead to new strategies to improve islet transplant engraftment and ensure immune protection of the grafts.
Because of its strategic positioning at the interface between the graft and circulating immune cells, the biological characteristics of the endothelium forming new blood vessels during tissue repair are of primary importance to the survival of transplants. We have identified a subset of human cord blood-derived hemopoietic progenitors that exhibits the developmental potential and functional properties of true endothelial progenitor cells through engagement of angiopoietin-dependent signals. Using animal models, we have further defined unique developmental, phenotypic and functional characteristics of bone-marrow-derived versus tissue-derived endothelial cells in islet transplant settings. Currently, this line of research aims to define the survival/proliferative/ differentiative signals delivered by bone marrow-derived vascular cells to islet tissue, and their involvement in modifying innate immune responses during tissue repair. Understanding these issues may lead to new strategies to improve islet transplant engraftment and ensure immune protection of the grafts.
Identify developmental cues provided by myeloid immune cells to pancreatic progenitors and exploit these cues for pancreatic islet cell regeneration.
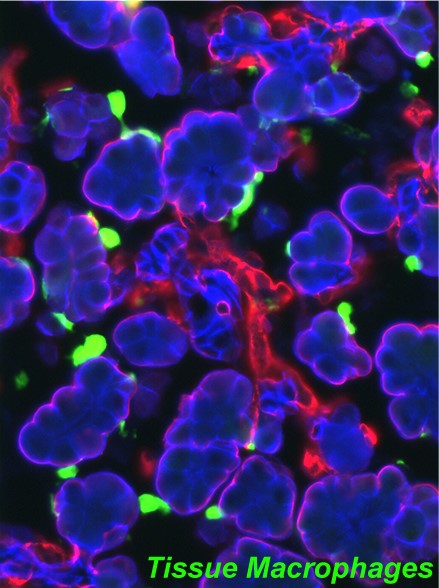 Tissue macrophages populate the mesenchymal compartment of all organs during embryogenesis and have been shown to support tissue organogenesis and regeneration. Nevertheless, the current dogma in the field is that their function(s) is peripheral to cell-autonomous epithelial development, i.e. being mainly restricted to clearance of apoptotic cells and remodeling of the extracellular matrices. By using organ culture and in vivo models, we found instead that macrophages in select states of activation directly influence the transcriptional program of pancreatic epithelial progenitors. Using myeloid cell subset-specific depletion models, we further discovered that myeloid cells marked by the expression of CCR2 are critically required for the establishment of the islet cell mass in perinatal life and islet regeneration in adulthood after injury. Gene profiling of these myeloid cell populations has pointed to candidate pro-regenerative pathways currently under investigation.
Tissue macrophages populate the mesenchymal compartment of all organs during embryogenesis and have been shown to support tissue organogenesis and regeneration. Nevertheless, the current dogma in the field is that their function(s) is peripheral to cell-autonomous epithelial development, i.e. being mainly restricted to clearance of apoptotic cells and remodeling of the extracellular matrices. By using organ culture and in vivo models, we found instead that macrophages in select states of activation directly influence the transcriptional program of pancreatic epithelial progenitors. Using myeloid cell subset-specific depletion models, we further discovered that myeloid cells marked by the expression of CCR2 are critically required for the establishment of the islet cell mass in perinatal life and islet regeneration in adulthood after injury. Gene profiling of these myeloid cell populations has pointed to candidate pro-regenerative pathways currently under investigation.
Engineer islet-like tissue microenvironments to enhance transplant engraftment in vivo, and to model diabetogenic insults and therapies ex-vivo.
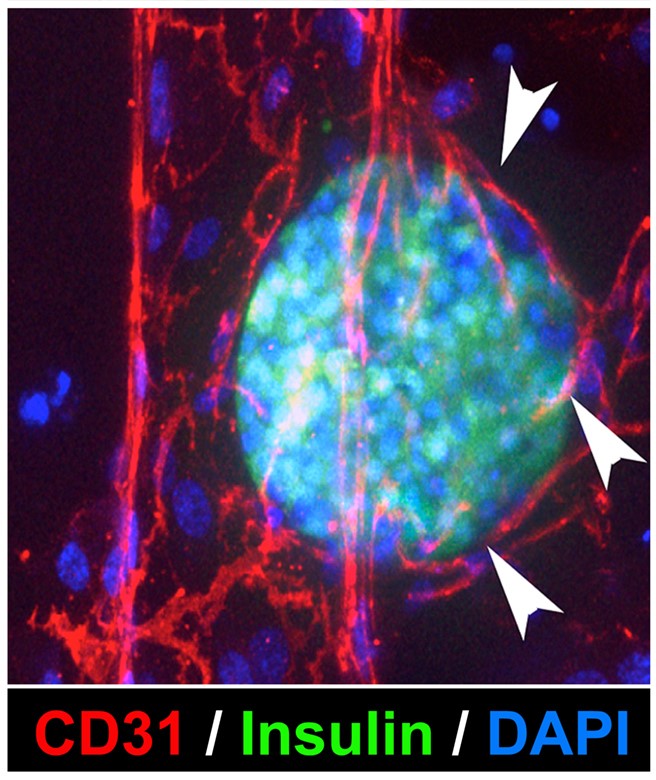 Since her appointment at the University of Washington, together with other members of the DOCE and ISCRM, Dr. Crisa has been engaged in efforts to develop a Diabetes Stem Cell Program, also part of the Institute of Stem Cell and Regenerative Medicine. Over the past years, our team has generated several pluripotent stem cell lines from different genetic backgrounds, and distinct cell types, and is now investigating unique signature pathways that dictate their ability to engraft in immune competent hosts, with a primary focus on those involved in the recruitment of vasculogenic cells and/or in the modulation of immunoregulatory networks. More recently, in collaboration with bioengineers, we have designed in vitro organ model systems that, thanks to biochemical patterning and microfluidic technologies, can emulate in vivo-like tissue microenvironments. Our medium and long-term plan is to further develop these model systems to mimic key aspects of pancreatic islet physiology and pathology, as well as to exploit these systems as a drug-screening platform to assess efficacy and safety of therapeutics.
Since her appointment at the University of Washington, together with other members of the DOCE and ISCRM, Dr. Crisa has been engaged in efforts to develop a Diabetes Stem Cell Program, also part of the Institute of Stem Cell and Regenerative Medicine. Over the past years, our team has generated several pluripotent stem cell lines from different genetic backgrounds, and distinct cell types, and is now investigating unique signature pathways that dictate their ability to engraft in immune competent hosts, with a primary focus on those involved in the recruitment of vasculogenic cells and/or in the modulation of immunoregulatory networks. More recently, in collaboration with bioengineers, we have designed in vitro organ model systems that, thanks to biochemical patterning and microfluidic technologies, can emulate in vivo-like tissue microenvironments. Our medium and long-term plan is to further develop these model systems to mimic key aspects of pancreatic islet physiology and pathology, as well as to exploit these systems as a drug-screening platform to assess efficacy and safety of therapeutics.
Current and Recent Lab Members

Laura Crisa, MD, PhD
Principal Investigator, Associate Professor of Medicine
Laura Crisa received her M.D. from the University of Rome in Italy in 1986, Board Certification in Endocrinology and Metabolism in 1989, and PhD in Immunology in 1992. Soon after her medical training, she joined the laboratory of Dr. Aldo Rossini, at the University of Massachussets, Boston, to train as a post-doctoral fellow in the field of Immunology and animal models of Type 1 diabetes. In 1994 she moved to the Scripps Research Institute, in La Jolla, as Senior Research Associate to further train in hemopoiesis and vascular biology, as applied to the biology of pancreatic islet transplantation. At the Scripps Research Institute she was appointed to the faculty in 2002. From there in 2009, she was recruited as an Associate Professor to the University of Washington within the Diabetes and Obesity Center of Excellence and the Institute of Stem cell and Regenerative Medicine.
Jessica O'Sell
Research Associate I
Research Interests: Molecular Biology, animal models of diabetes.
Stephanie Pardike
Research Associate I
Research Interests: Animal models of diabetes, genetic screening of imprinted genes.
Kristine Mussar
Graduate Student
Research Interests: Macrophage regenerative functions, stem cell biology.
Vincent So
Summer Student, CIIID Scholar
Research Interests: Mechanisms of gene imprinting in macrophages.
Addie Gerhart
Undergrad Student
Research Interests: Macrophage function, Pancreatic Islet regeneration.Contact Us
UW Medicine Diabetes Institute
850 Republican Street, Box 358062
Seattle, WA 98109-8062
Laura Crisa Office: (206) 685-7815
Laboratory: (206) 616-9782
Laura Crisa Email: lcrisa@uw.edu
To inquire about Postdoctoral and Graduate Student Openings click on: lcrisa@uw.edu


