| 3. |
Shown below is the transcription activation pathway for Gene X, needed for entry into S phase. Binding of growth factor to the receptor (Protein 1) causes the receptor to phosphorylate Protein 2. As a result, Protein 2 releases Protein 3, which is then free to enter the nucleus and activate transcription of Gene X.
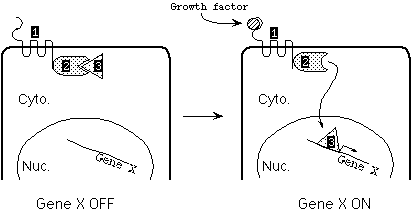
|
|
(a)
|
Proteins 1 through 3 are encoded by Genes 1-3. For each gene:
| (i) |
is a mutation (any random mutation) more likely to result in an allele that promotes cancer, or in a non-cancer allele? Explain. |
| (ii) |
what kind of mutation in that gene would result in an allele that is more likely to promote progression toward cancer -- a gain-of-function mutation or a loss-of-function mutation.
|
| (iii) |
Would the cancer-promoting allele be dominant or recessive? Assume that each gene product is normally present in excess. |
|
|
(b) |
Suppose that mutation of a certain locus is the final step in tumorigenesis in a certain cell. If the chance of oncogenic mutation is 10-5
per allele of a gene , what is the chance that the cell (wild type for that gene) will become a cancer cell if the gene is (i) a proto-oncogene? (ii) a tumor suppressor gene? |
| 4. |
Wild-type Rb protein can bind to protein E2F, sequestering it in the cytoplasm. When Rb protein is phosphorylated, it releases E2F, allowing E2F to enter the nucleus, where it can activate transcription of genes needed for entry into S phase. |
|
(a) |
In cells where one copy of the Rb gene has been deleted and the other copy is normal, do you expect E2F to be sequestered in the cytoplasm or in the nucleus (assuming no phosphorylation of Rb)? |
|
(b) |
In cells homozygous for deletion of the Rb gene, do you expect E2F to be sequestered in the cytoplasm or in the nucleus? |
|
(c) |
Suppose a cell is heterozygous for deletion of the Rb gene and for deletion of the E2F gene. Do you expect E2F in this cell to be sequestered in the cytoplasm or in the nucleus? |
|
(d) |
Suppose the cell in (d) undergoes additional mutations, so that it becomes homozygous for loss of the Rb gene and homozygous for loss of the E2F gene. Would you consider this cell to be in increased danger of progressing towards cancer? Explain. |
|
(e) |
Suppose a different cell is heterozygous for a mutation in Rb such that the resulting protein remains bound to E2F even after phosphorylation. Would you consider this cell to be in increased danger of progressing towards cancer? Explain. |
| 8. |
This one is a modified exam question from 1998.
You have been working with two mice, Constance and Big Bertha, that show a high incidence of aborted fetuses in crosses with normal males. Constance had been X-irradiated when she was an embryo, and Big Bertha had been been exposed to a chemical mutagen when she was an embryo. The data you have collected on the aborted fetuses are:
|
Mother |
Sex of aborted fetuses |
Karyotype of aborted fetuses |
|
Constance |
Female |
No Barr bodies |
|
Big Bertha |
Female and male |
All X chromosomes form Barr bodies |
|
|
(a) |
Explain why:
- all of Constance's daughters die
- all of Constances's sons survive
- all of Big Bertha's offspring die
[Note: This part is not asking about what defect the X-ray/mutagen treatment has caused; the question is only asking about what makes the offspring die or not die.]
|
|
(b) |
Explain how the X-ray treatment or mutagen treatment might have caused the phenotypes described above. In particular, what part of the X chromosome might be affected in each case, and how might that effect cause the observed phenotypes? |
|
(c) |
A third female mouse (Chimney) showing high frequency of aborted fetuses was found subsequently. She too had been exposed to X-rays when she was an embryo. In her case, the aborted fetuses sometimes had a condensed, Barr body-like form of one chromosome 14 homolog. Briefly explain how this state might come about. Would you expect to see a male- or female-specific lethality in this case? Why, or why not? |
