Blood activates Vamp gene transcription. Therefore, blood is either an activator of a transcriptional activator (just as cAMP activates CAP in E. coli), or it represses a transcriptional repressor (just as lactose represses the lac repressor).
Since the homozygous B mutant shows constitutively high transcription whether blood is present or absent, the B protein must be this activator or repressor. So now we have to determine whether it's an activator or a repressor, and we can do that by looking the mutant phenotype to see the mutant phenotype is dominant or recessive (just as we did for the GAL4/GAL80 system):
- If B is an activator of Vamp transcription, the B- mutant must be a gain-of-function mutation that activates transcription even in the absence of blood.
- However, if B is a repressor, then the B- mutation is expected to be a loss of function allele (the mutant repressor cannot block Vamp gene transcription, so the presence or absence of blood becomes irrelevant).
The B+/B- heterozygote shows low transcription in the absence of blood--so the B- phenotype is recessive, loss-of-function, supporting the repressor model (B is a repressor of Vamp gene transcription, and that the repressor is itself repressed by blood). Repression can act either directly on the Vamp gene, or indirectly through gene G (see below).
What can we tell about the garlic response?
- Garlic blocks Vamp gene transcription--so it either activates a repressor or blocks an activator (gene G).
- Furthermore, garlic still blocks transcription in the repressorless B-/B- mutant, suggesting that regulation of transcription by garlic is either independent of the B gene pathway of regulation, or that gene G acts downstream of gene B (is epistatic to B).
- The G-/G- mutant shows constitutively low transcription under all conditions, so the mutant protein is either a dominant, gain-of-function repressor (it permanently represses and is resistant to garlic), or a recessive, loss-of-function activator (in the absence of activator, there is no transcription, so the presence or absence of garlic becomes irrelevant).
Again, the G+/G- heterozygote indicates that the mutant phenotype is recessive. Therefore, protein G must be an activator of transcription and is repressed by garlic. In wildtype flies, Vamp gene transcription occurs only when blood is present (to remove the repressor, B) and in the absence of garlic (allowing activation by the activator, G).
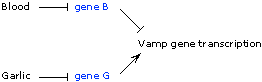
Alternatively, if B and G act through the same pathway, then G acts downstream of B and is repressed by B.
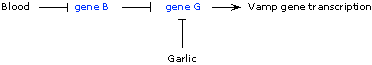
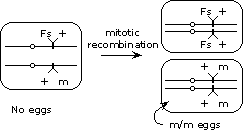 To get around this problem, we can induce mitotic recombination (using the X-ray source) in developing females that are heterozygous (m/+) for the maternal effect gene. In some of the females, mitotic recombination between the centromere and the gene will produce a clone of cells that are homozygous for the mutation in the tissues that produce the eggs (assuming that development of this tissue is not one that requires the gene to be functioning), thereby allowing the maternal effect phenotype to be manifested.
To get around this problem, we can induce mitotic recombination (using the X-ray source) in developing females that are heterozygous (m/+) for the maternal effect gene. In some of the females, mitotic recombination between the centromere and the gene will produce a clone of cells that are homozygous for the mutation in the tissues that produce the eggs (assuming that development of this tissue is not one that requires the gene to be functioning), thereby allowing the maternal effect phenotype to be manifested.