|
Genetics 371B, Autumn 1999
Answer key, Problem set 4 |
| 1. |
The main point to note here is that, as discussed on p.67 of lecture
notes, having an odd number of crossovers within a paracentric
inversion (in heterozygotes) will result in recombinant gametes
that are either dicentric or acentric and are therefore inviable--causing
an apparent reduction in recombination frequency. Likewise, deletions
have the effect of reducing recombination frequency in heterozygotes.
Inversion homozygotes don't have the problem that heterozygotes
do, and the recombination frequency there will be unaffected.
[Note: with inversions, even-numbered crossovers can give viable
recombinant gametes. However, the question only asks for the recombination
frequency between Hs and D. If we are looking only at Hs and D, even numbers of crossovers wouldn't be scored as recombinants
anyway--only odd-numbered crossovers would give recombinant products.
Therefore, we can ignore the contribution of viable products arsing
from double crossovers.] |
|
(a) |
The inversion involves 40% of the full length (400 kb out of 1000
kb). Therefore, only 60% of the recombinant products will be viable;
the apparent recombinant frequency will be (0.6)*(20) = 12%. (Another
way of thinking about it -- only the 600 kb outside of the inversion
is available for productive recombination.)
[Addendum: This is an approximation -- we expect that 12% of total meiotic products will be recombinant in the "allowed" intervals.
But because products of recombination in the loop are lost from
the pool of viable products, the percent viable recombinants will
be a little higher than 12%. Thanks to Jimmy Liu and Joon Um for
pointing this out.] |
|
(b) |
As described above, homozygotes will have no trouble giving viable
recombinant progeny -- the recombinant frequency will be unchanged
at 20%. |
|
(c) |
Here, 10% of the distance is missing, so the recombinant frequency
will be 90% of the original: (0.9)*(20) = 18%. |
| 2. |
Having only one sex chromosome has different phenotypic effects
than having two -- even when one of them is largely silenced --
therefore, it follows that the second X chromosome in an XX female
cannot be fully silenced all of the time. In fact, we know that
it is not -- |
|
(a) |
Some 15 or 20 genes on the silenced X (predominantly in the pseudoautosomal
region, which shares homology with the Y chromosome) are known
to be actively transcribed. Having only one X chromosome (as in
Turner syndrome patients) must alter the dosage of these gene
products, perhaps with deleterious consequences. In males, the
corresponding genes might be present in the pseudoautosomal region,
allowing gene dosage to be maintained. Alternatively, the effects
of the XO genotype may be deleterious only with respect to female
sex differentiation. |
|
(b) |
X chromosome silencing normally does not occur until after the
first several cell divisions in embryogenesis. Having two copies
of the X chromosome present and active for those first few divisions
might be important for the female developmental program. |
| 3. |
The question here is, in a cell with a normal allele and a mutant
allele, which phenotype will the cell display -- mutant or wild
type? [If it helps, think about the driving-school car analogy,
where the tumor suppressor -- Rb -- is analogous to the brake
pedal and the protooncogene -- E2F -- is like the accelerator,
and there are two brake pedals and two accelerator pedals.] |
|
(a) |
(i) |
The phenotype of a mutant Rb that cannot be phosphorylated is
that it will never release E2F, and will therefore not allow cell
proliferation. This phenotype is expected to be a dominant, gain-of-function
mutation -- the normal Rb molecules can get phosphorylated and
thereby release E2F, but because there is an excess of Rb, those
freed E2F molecules will promptly get bound up by the mutant Rb.
|
|
|
(ii) |
The mutant phenotype is that E2F will always be released, allowing
S phase. However, because there is still plenty of normal Rb around,
the E2F molecules will be bound by the normal Rb, so the mutant
phenotype (E2F never bound) will not be seen. |
|
|
(iii) |
Assuming that mutant, phosphorylated Rb is capable of binding
fresh E2F,this mutation will behave just like the mutation in
(i), and will therefore be dominant. |
|
|
(iv) |
A dominant mutation -- even if the normal E2F molecules are getting
bound by Rb, the mutant ones can trigger cell proliferation, so
the mutant phenotype will always be seen. |
|
|
(v) |
A recessive mutation: the phenotype of the mutant form of E2F
is that it cannot be released and therefore, it cannot trigger
S phase -- but the normal E2F can be released and will trigger
S phase (when appropriate), so the normal phenotype is dominant. |
|
|
(vi) |
Again, the mutant phenotype is no S phase -- but the normal allele
is capable of triggering S phase, so the normal phenotype is dominant
and the mutant is recessive. |
|
(b) |
Only the mutations that promote cell proliferation (E2F escapes
Rb control) increase the risk of cancer. Of the mutations discussed
above, only two -- (ii) and (iv) have such a property. (In all
other mutations, the phenotype is in the other direction -- S
phase is not allowed. Therefore the other mutations do not increase
the probability of cancer.) |
| 4. |
Because we are dealing with small patches of recessive phenotypes
against a background of the dominant phenotypes, this is obviously
a question involving mitotic recombination. Note here that while
black and dry phenotypes can be seen in skin patches, the Jedi phenotype cannot--the Jedi phenotype involves the whole animal. The presence of the Jedi phenotype is specific cells has to be inferred from the phenotypes
arising from loci linked to Jedi. |
|
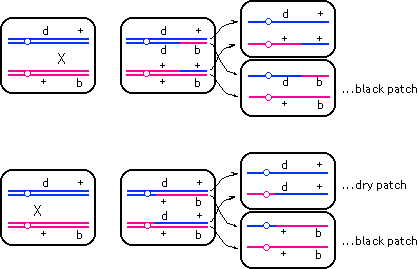
(a) |
Because we are seeing twin patches here, the two dominant alleles
must be in the trans configuration. Furthermore, there are plenty
of lone black patches but the dry patches are never solitary --
so the black gene must be further from the centromere than the
dry gene (d is closer to the centromere). Lone black patches are the result
of a crossover between b and d, while the twin patches result from a crossover between the centromere
and d.
|
|
(b) |
The lone black patches may occasionally be homozygous for J -- but that would occur only if the crossover was between J and the centromere. The twin patches give a better probability
of getting JJ homozygous cells. The twin patches arise from single crossovers
between the centromere and d as shown above. Because allele J is originally on the same chromatid as allele b, and because the crossover does not separate these two alleles,
J will continue to segregate with b -- and therefore, cells that are bb (black patches next to dry patches) will likely be JJ also, barring the remote possibility of a triple crossover (see
part (c) below); the dry patches will be homozygous normal for
J.
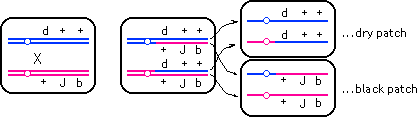
|
|
(c) |
The only way to get dry and black twin patches without also making
J homozygous is if J were separated from b by a crossover. A triple crossover could give the observed result
--
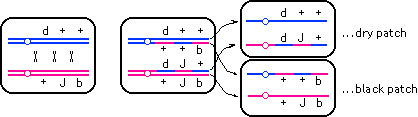
|


