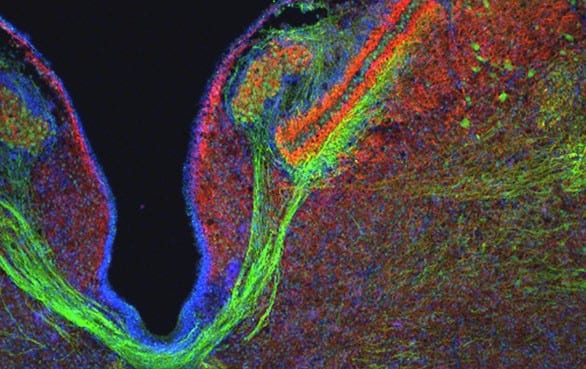
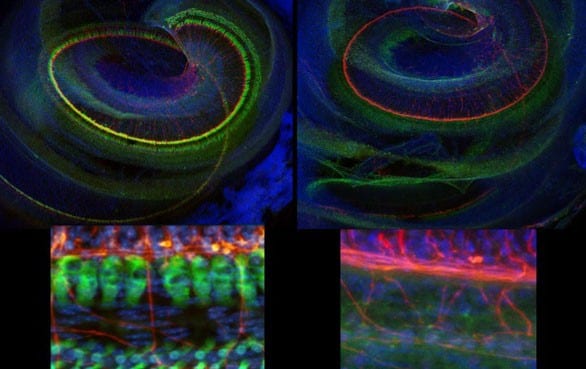
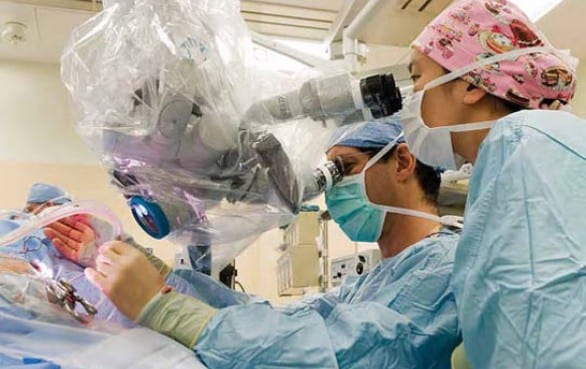
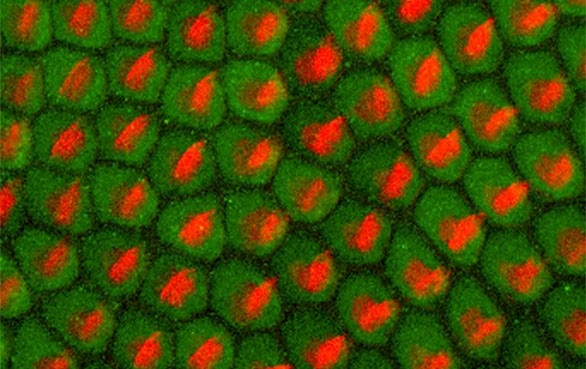
The Virginia Merrill Bloedel Hearing Research Center is located on the campus of the University of Washington. Founded in 1988 with a generous donation from Prentice and Virginia Bloedel, the Center is one of the nation’s leading sites for research and education related to hearing, hearing loss, balance, disequilibrium, and related communication disorders. The Center is associated with the Department of Otolaryngology – Head and Neck Surgery in the School of Medicine, has a strong affiliation with the Department of Speech and Hearing Sciences, and through 73 faculty Bloedel Affiliates is connected to 18 academic departments across the University of Washington.
The Bloedel Center is a communication focal point among basic and clinical scientists to facilitate the sharing of ideas and information for the collective advancement of the Bloedel Mission. State of the art facilities for molecular biology, tissue culture, microtomy, histology, cellular imaging, and vestibular and auditory testing are available. The Center supports the Seminars in Hearing and Communications Sciences lecture series, which sponsors speakers from around the world, awards competitive Mini-Grants to Bloedel Affiliates for pilot studies that lead to full grant submissions, and finances a Traveling Scientist program, which encourages meaningful collaborations with other research institutions throughout the world.
Through research, collaboration, teaching, and innovation, the Virginia Merrill Bloedel Hearing Research Center focuses continually on its mission of “Working together so all might hear”.