Fast-scan cyclic voltammetry
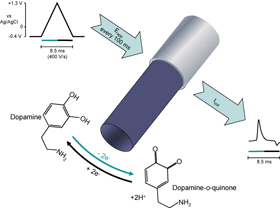
Voltammetric detection of dopamine. When sufficient potential is applied to the electrode, dopamine is oxidized to dopamine-o-quinone, donating two electrons that are detected as current. When the potential is returned, any dopamine-o-quinone remaining at the electrode surface is reduced back to dopamine by accepting electrons, producing current in the opposite direction. In the example shown, the potential is applied by fast-scan cyclic voltammetry. With this technique, the resultant current comprises time-resolved peaks that aid analyte identification. These measurements are typically repeated several times per second.
Using fast-scan cyclic voltammetry at carbon-fiber microelectrodes, changes in the extracellular concentration of electroactive molecules can be monitored. One such molecule that is of biological interest is the neurotransmitter, dopamine (see figure). The potential at the microelectrode is held at a potential insufficient to oxidize dopamine (e.g., -0.4 V vs a Ag/AgCl reference electrode) and then linearly ramped to an oxidizing potential (e.g., +1.3 V) and back at a high scan rate (e.g., 400 V/s) multiple times each second. When dopamine is present in the solution at the surface of the electrode, it is oxidized during the positive sweep to form dopamine-o-quinone (peak reaction at approximately +0.7 V) which is reduced back to dopamine in the negative sweep (peak reaction at approximately -0.3 V). During the redox reactions electrons are transferred between these molecules and the microelectrode (electrolysis). This flux of electrons is measured (as current) and is directly proportional to the number of molecules that undergo electro-oxidation. For analyte identification, current during a voltammetric scan can be plotted against the applied potential to yield a cyclic voltammogram. The cyclic voltammogram provides chemical information that is fairly unique for each substance and thus allows resolution of dopamine from other electroactive compounds. For quantification of changes in dopamine concentration over time, the current at its peak oxidation potential can be plotted for subsequent voltammetric scans. This approach can be utilized to make rapid chemical measurements in a range of biological preparations and conditions. We have developed this technology to allow us to record dopamine in real time, in awake rodents engaging in a variety of behavioral tasks.
References
Single cells
Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis
Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ Jr, Near JA and Wightman RM
Journal of Biological Chemistry 265, 14736-14737 (1990)
Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells
Kozminski KD, Gutman DA, Davila V, Sulzer D and Ewing AG
Analytical Chemistry 70, 3123-3130 (1998)
Dopamine transport into a single cell in a picoliter vial
Troyer KP and Wightman RM
Analytical Chemistry 74, 5370-5375 (2002)
Brain slices
Application of fast cyclic voltammetry to measurement of electrically evoked dopamine overflow from brain slices in vitro
Bull DR, Palij P, Sheehan MJ, Millar J, Stamford JA, Kruk ZL and Humphrey PPA
Journal of Neuroscience Methods 32, 37-44 (1990)
Detection of dopamine overflow and diffusion with voltammetry in slices of rat brain
Kelly RS and Wightman RM
Brain Research 423, 79-87 (1987)
Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing patterns
Phillips PEM and Stamford JA
Brain Research 884, 139-146 (2000)
Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum
Zhou FM, Liang Y and Dani JA
Nature Neuroscience 4, 1224-1229 (2001)
Properties of dopamine release and uptake in the songbird basal ganglia
Gale SD and Perkel DJ
Journal of Neurophysiology 93, 1871-1879 (2005)
Anesthetized animals
Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle
Millar J, Stamford JA, Kruk ZL and Wightman RM
European Journal of Pharmacology 109, 341-348 (1985)
Dopaminergic neurons: simultaneous measurements of dopamine release and single-unit activity during stimulation of the medial forebrain bundle
Kuhr WG, Wightman RM and Rebec GV
Brain Research 418, 122-128 (1987)
Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool
Venton BJ, Seipel AT, Phillips PEM, Wetsel WC, Gitler D, Greengard P, Augustine GJ and Wightman RM
Journal of Neuroscience 26, 3206-3209 (2006)
Freely-moving animals
Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats
Budygin EA, Phillips PEM, Robinson DL, Kennedy AP, Gainetdinov RR and Wightman RM
Journal of Pharmacology and Experimental Therapeutics 297, 27-34 (2001)
Dynamic gain control of dopamine delivery in freely moving animals
Montague PR, McClure SM, Baldwin PR, Phillips PEM, Budygin EA, Stuber GD, Kilpatrick MR and Wightman RM
Journal of Neuroscience 24, 1754-1759 (2004)
Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats
Cheer JF, Wassum KM, Heien MLAV, Phillips PEM and Wightman RM
Journal of Neuroscience 24, 4393-4400 (2004)
Behaving animals
Subsecond dopamine release promotes cocaine seeking
Phillips PEM, Stuber GD, Heien MLAV, Wightman RM and Carelli RM
Nature 422, 614-618 (2003)
Dopamine operates as a subsecond modulator of food seeking
Roitman MF, Stuber GD, Phillips PEM, Wightman RM and Carelli RM
Journal of Neuroscience 24 1265-1271 (2004)
Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals
Clark JJ*, Sandberg SG*, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N and Phillips PEM
Nature Methods 7, 126-129 (2010)
Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine
Gan JO*, Walton ME* and Phillips PEM
Nature Neuroscience 13, 25-27 (2010)
Reviews
Probing brain chemistry
Stamford JA and Justice JB Jr
Analytical Chemistry 68, 359A-363A (1996)
Critical guidelines for validation of the selectivity of in-vivo chemical microsensors
Phillips PEM and Wightman RM
Trends in Analytical Chemistry 22, 509-514 (2003)
Detection technologies. Probing cellular chemistry in biological systems with microelectrodes
Wightman RM
Science 311, 1570-1574 (2006)


