Smoke
Infusion for Seed Germination
in Fire-adapted Species
Daniela Shebitz, Anne Andreu, Marlo Mytty, Doug Schmitt, and Mike Cooksey
Introduction
Numerous species that
inhabit fire-dependent ecosystems have evolved reproductive strategies to adapt
to factors associated with fire (Van Staden et al. 2000). These adaptations are particularly evident in
seeds that respond to the physical (i.e. temperature and light) and/or chemical
(smoke, gas, nutrients) germination cues associated with fire. In fact, many species have evolved barriers
to seed germination that are overcome only by fire-related cues (Keeley
1998).
Seeds of many species
germinate in response to physical signals associated with fire, such as
fracturing or desiccation of the seed coat by heat (Jeffrey et al. 1998). Heat may also stimulate the embryo directly
(Blommaert 1972). For a substantial
number of species with fire-triggered germination, however, chemicals from
combustion induce germination, not the heat (Keeley 1998).
In western
In this paper, we will first
present the effects that smoke has on seed germination, discuss why this
relationship can be incorporated into habitat restoration, and provide
information on species and ecosystems that can potentially benefit from smoke
technology. We will then discuss various
methods of incorporating smoke technology into restoration, explaining in
detail our method of choice.
General effects of smoke on germination
Smoke is clearly one of the
products generated as a consequence of fire.
There is no evident indication of the mechanisms by which smoke affects
germination. It is known, however, that the chemical signals of smoke not only
influence seeds during fires and in the immediate post-fire environment, but
the signals last for considerable periods after the fire, and perhaps most
importantly, can travel to communities long distances away from the fire (Van
Staden et al. 2000).
Due to the fact that smoke
particles can adhere to plant surfaces, persist in the soil, and be adsorbed to
soil particles, smoke particles have major effects on scarified seeds in the
soil (Van Staden et al. 2000).
Egerton-Warburton (1998) demonstrated that this ability of smoke to
adhere to soil and plant surfaces plays a role in the germination process by
changing the morphology of the seed and causing an intense chemical
scarification of the seed surface.
Roche et al. (1997) found
that some species respond only to smoke application to the soil seed bank, and
not to the application of smoke to freshly collected seed. The authors suggest that some seeds need to
enter the soil seed bank before they are receptive to the germination-promoting
effects of smoke.
In some species, such as Erica
sessiliflora, smoke treatment on seeds can substitute for a light
requirement. Such a response, which was
also observed for light-sensitive Grand Rapids lettuce seeds (Drewes et al.
1995), makes seedling recruitment more probable if smoke dissolved in water
penetrates into the soil. This
characteristic ensures that even in the dark, there will be some germination of
light-sensitive seeds in the absence of major soil disturbance (Van Staden et
al. 2000).
Until recently, the role of
chemical cues in seed germination received little attention (Van Staden et al.
2000). In addition to heat, vegetation
fires release chemical cues such as ethylene and ammonia. While both of these gases are known to
stimulate germination, it has been shown that ethylene is not the active
compound in smoke solutions that stimulates germination. (Jager et al.
1996). Numerous studies have attempted
to determine the chemical components responsible for charred wood and
smoke-stimulated germination, but have not successfully identified the active
components (Keeley 1998).
Smoke technology in habitat restoration
With increased urbanization,
fire as a restoration tool is not always feasible. Smoke technology provides the ability to
incorporate the chemicals associated with fire in to a restoration when fire is
not possible. There are numerous
benefits of incorporating smoke into a habitat restoration project. Below are some examples that were presented
by Van Staden et al. (2000):
- Testing seed viability – Smoke and smoke extracts can test the
viability of seeds from soil seed banks in areas that have become invaded
by exotic plant species.
Germinating the seeds using smoke will assist in determining
whether a viable reserve of native species seeds that benefit from fire still
exists in the area.
- Giving existing native species an “edge” - Physically removing exotic species and then
smoking the soil using smoke tents or applying aqueous smoke solutions may
assist in restoring native plants.
- Stimulate germination of native seeds in the
existing soil seed bank – If
there are native species in an area that has been characterized by
frequent fires in the past, you can stimulate the germination of seeds by
smoking the soil (see 2).
- Pretreating broadcast seed with aerosol smoke to
increase the number of germinants
– Compared to unsmoked seed, pretreatment of broadcast seeds with aerosol
smoke has been found to result in significant increases in the total
number of germinants and responding species.
- Germinating seeds that are otherwise difficult
or impossible to germinate –
Many wildflower species in the families Asteraceae, Bruniaceae, Ericaceae,
Thymelaeaceae, and Restionaceae that responded to smoke were previously
difficult or impossible to germinate in a nursery.
- Removing the need for further dormancy-breaking
treatments before sowing –
Numerous plants such as vegetable crops have a potential to be primed with
aqueous smoke extracts and then stored for later use.
- Flexibility in scheduling – Seeds may be smoke-treated immediately before
sowing, or they may be treated prior to sowing and then dried and stored.
- Protection for seeds – Roche et al (1997) suggest that high levels of
smoke by protect seeds against predation and microbial attack.
Species and ecosystems
that can benefit from smoke technology
Many species, especially
those from fire adapted ecosystems, respond to germination cues from heat,
smoke, or a combination of the two. Since research in this area is fairly new,
isolation of the specific mechanisms by which germination is stimulated are
often not known. Species that have been found to respond to heat do so because
of “heat shock”. Species that have been
studied are “hard-seeded”, with a prominent waxy cuticle and dense palisade
layer of sclerids that enforces dormancy by making the seed coat impermeable to
water. Brief heat shock between 80° and 120° C is sufficient to cause the seed
to imbibe water by loosening cells or possibly denaturing germination
inhibitors. For some species, heat shock alone may work, but some heat-induced
species also require light and/or cold stratification. Heat-shock germination
is widespread in the following families: Fabaceae, Rhamnaceae (includes Ceanothus),
Convolvulaceae, Malvaceae, Cistaceae, and Sterculiaceae and is found in many
ecosystems. (Keeley 1998).
Other fire-evolved species
have been found to respond not to heat, but to chemicals from combustion -
either from smoke or in charred wood. Compared to heat shock, little is known
regarding which chemicals stimulate germination and how. Charred wood has been shown to stimulate
germination in the species Emmenanthe penduliflora, a
Table 1.
Chaparral species demonstrating highly statistically significant
smoke-induced germination. Seeds of all annual species were collected in
southern
|
Family |
Species |
Growth Form |
|
Asteraceae |
Chaenactis
artemisiifolia |
Annual |
|
Boraginaceae |
Cryptantha clevelandi |
Annual |
|
|
C. micrantha |
Annual |
|
Brassicaceae |
Caulanthus
heterophyllus |
Annual |
|
Caryophyllaceae |
Silene multinervia |
Annual |
|
Hydrophyllaceae |
Emmenanthe penduliflora |
Annual |
|
|
Eucrypta
chrysanthemifolia |
Annual |
|
|
Phacelia grandiflora |
Annual |
|
|
P. minor |
Annual |
|
Lamiaceae |
Salvia apiana |
Shrub |
|
|
S. columbariae |
Annual |
|
|
S. leucophylla |
Shrub |
|
|
S. mellifera |
Shrub |
|
Loasaceae |
Mentzelia micrantha |
Annual |
|
Onagraceae |
Camissonia californica |
Annual |
|
Papaveraceae |
Romneya coulteri |
Suffrutescent* |
|
Polemoniaceae |
Allophyllum glutinosum |
Annual |
|
Scrophulariaceae |
Antirrhinum
coulterianum |
Annual |
|
|
A. kelloggii |
Annual |
|
|
A. nuttallianum |
Annual |
|
|
Mimulus clevelandii |
Suffrutescent* |
|
|
Penstemon
centranthifolius |
Suffrutescent* |
|
|
|
|
|
* Suffrutescent = herbaceous with woody caudex. |
||
Source: Keeley 1998.
Experiments performed by
Keeley (1998) found that the length of exposure to smoke was very important in
some species - a 3 minute difference in exposure resulted in the death of some
seeds. Some closely fire-linked species
didn’t germinate under heat or smoke treatments alone. In some cases burial for one year or cold
stratification are required in addition to smoke exposure. All of these factors can have an effect on
germination and should be considered when using smoke or chemicals in smoke to
induce germination.
Structurally, there are
characteristics shared by smoke-stimulated species found by Keeley (1998). For
most species, the outer cuticle was weakly developed and the exterior of the
testa highly sculptured, in contrast to the smooth character of Ceanothus
and many heat-stimulated seeds. For an in-depth discussion of the difference
between seeds that are heat and smoke-stimulated, see Keeley (1998).
Blank and Young (1998)
showed the following sagebrush-steppe species in the western
In addition to those listed
in the introduction, other ecosystems for which smoke (or chemicals from smoke)
has been successful in inducing germination of native plants include:
Mediterranean-type ecosystems, western Australia ecosystems, for which Roche et
al. (1997) reported increased germination on 75 new species, and specifically
dry sclerophyll spotted gum (Corymbia maculata) forest communities in
Techniques for smoke
pre-treatments of seeds for use in restoration
There are two basic methods
for exposing seeds to smoke or the chemicals derived from smoke that are
thought to promote germination in many seeds.
The first is to expose seeds directly to smoke and the other is to
indirectly expose seeds to the particulates of smoke through the use of “smoke
water” or smoke distillates in a dry form. Multiple ways to approach both of
these seed exposure techniques exist.
Direct seed exposure to smoke:
·
Place clean, dry
seeds on permeable trays, mesh racks or petri dishes and place them in a poly
tent. Burn native vegetation in a metal
drum adjacent to the tent and use a fan or compressed air to blow cooled smoke
created by the fire into the poly tent through a long plastic pipe. Using a long pipe allows the smoke to cool
before entering the tent. The optimum
exposure time is variable for different seeds but 1 hour is common. (Tieu et
al. 2001)
·
Sow seeds in
nursery flats in a soil-less potting mix.
Place the flats in a poly tent and follow the same directions as
above. Exposure time is generally 1-3
hours. After smoke exposure, spray the
flats with a fine mist of water to settle smoke particles on the soil surface
(Read et al. 2000).
·
Spread soil seed
bank samples on a thin layer of soil-less potting mix and follow the same
directions as above. Exposure time is
generally 1-3 hours. After smoke exposure, spray the area with a fine mist of
water to settle smoke particles on the soil surface (Read et al. 2000).
·
Force smoke into
low poly tents to treat soil seed bank in situ.
·
Force smoke into
a poly tent pitched over vegetation with ripe seeds that have not
dispersed. This is probably most
effective for smoke responsive shrub species (Greening Australia.).
When smoking seeds sown in
nursery flats or in situ, be careful not to over water in the first weeks or
until germination occurs. Over watering
will wash away smoke particulates, reducing effectiveness of the treatment.
(Greening
Indirect exposure to chemicals in
smoke:
·
Soak seeds in
smoke water (directions for making below).
A fish tank aerator can be used to minimize seed rotting.
·
Germinate seeds
on smoked filter paper.
·
Use smoked
filter paper in the presoaking of seeds.
Soak the filter paper in water to allow smoke particulates to diffuse
into water, then soak in the water while aerating.
·
Use commercially
available dry smoke products in the potting soil or native soil in which smoke
responsive seeds will be planted.
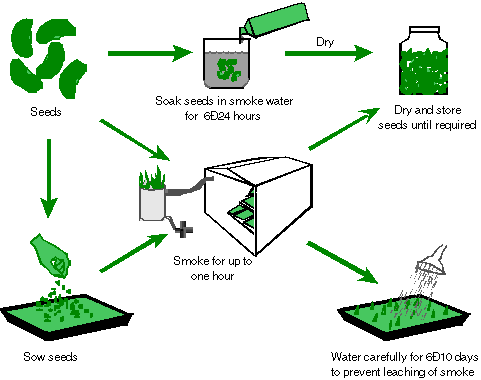
Figure 1. Various methods
for smoke application.
Smoke can be applied directly to seeds
or soils by:
Build a poly tent: Using PVC or metal poles build a tent frame
and cover with poly sheeting.
Build a smoke generator:
1. Make holes in the bottom and on the side near the top
of a large metal drum and attach piping to the side hole. Position the piping so that it enters the
bottom of the poly tent. Place green and
dry native woody and leafy vegetation into the drum and light it. Place a lid over the drum and blow air
through the bottom hole with a fan or compressed air. This air will feed the fire and force smoky
air out of the side hole, through the pipe and into the tent.
2. Using a beekeepers smoker, burn chipped or shredded
native vegetation as described above and blow the smoke into a small tent or
other small chamber (such as a chromatography chamber) (Morris 2000).
Place seeds on permeable
trays, petri dishes or sown seeds in flats inside the tent for 30 minutes to 1
hour (Tieu et al. 1999).
Smoke water can be created by:
1. Using the same drum technique described above, attach
the piping to another drum containing water.
Force the smoky air produced in the first drum through the water in the
second drum by using a small fan or compressed air.
2. Using a small grill, burn charcoal on half of the
base of the grill (as normal) and on the upper grill surface place a pan of
water on the other side and native vegetation (woody and leafy) on the side
above the charcoal. Cover the
grill. As the coals burn the native vegetation
the smoke that is created will be infused in the water in the pan. Be careful not to allow the water to boil
away. The water created in these ways
can be cooled and used immediately or frozen until needed.
3. Use commercially available smoke infused products:
Liquid
smoke
Smoke
infused paper discs
Dry
smoke infused material to add to planting medium: such as Regen 2000
In mine site rehabilitation
in
Testing Seed Responsiveness to Smoke
Treatments:
Before investing much
effort in creating smoke treatment facilities for restoration projects, it
would be wise to test the responsiveness of seeds to be used in the restoration
to smoke treatments.
Using sterile petri dishes
lined with sterile Whatcom filter papers, add distilled water or smoke water to
the dishes. If using distilled water,
use pre-smoke treated seeds to test the effectiveness of the treatment on
germination. If using smoke water, use
untreated seeds to test the effectiveness of smoke water treatment on
germination. Use control petri dishes
using untreated seeds and distilled water.
Place the dishes in a growth chamber or temperature/light controlled
greenhouse and examine regularly for evidence of germination (extension of
radicle from the seeds). (Brown 1992)
Recommended Technique for Incorporating
Smoke in Seed Germination
We have developed an apparatus which allows
for the germination of seeds through the use of:
1. Smoke (cooled)
2. Smoke water
3. Heat and smoke
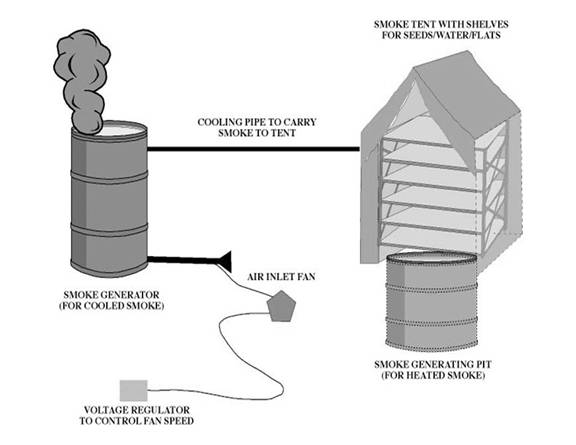
Figure 2. Our apparatus for germinating seeds by smoke,
smoke-water or heat.
The required materials for
creating and operating this apparatus are:
- poly tent with vents
- frame for tent
- 50 gallon drums (2)
- shovel to dig
- shelving (with 5 shelves)
- seed flats
- screens for seeds
- seeding mix
- trays for water
- voltage regulator
- air inlet fan
- cooling pipe
- tubing
- charcoal
- fire source (matches)
- native vegetation to burn for smoke
- electrical wiring
Heat germination
The apparatus that we have
developed is illustrated in Figure 2.
For those species that require heat to break the seed coat, a smoke
generating pit is located under-ground, under the smoke tent. Charcoal will be lit in the pit, and when the
flames die down, add native vegetation on the grill grate and then place the
seed trays in the tent. Within the smoke tent are shelves which, when heat is
incorporated, will hold trays with seeds sown in seeding mix. Alternatively, if you plan to sow the seeds
immediately following the heat/smoke germination, the seeds can be on screens,
and not be planted. For this use of the
tent, it is recommended that the bottom two shelves are not used, to avoid
over-heating. A thermometer is located
outside the tent that can read the temperature inside, and vents are located
throughout the tent to manipulate the heat within, and to control the amount of
smoke within the tent. We recommend
keeping the seeds in the tent for up to one hour if heat is incorporated.
Smoke and smoke-water germination
For those seeds are not
benefited by heat, the below-ground pit is not used. Instead, smoke is created in the above-ground
generator and is cooled through a pipe that connects the generator to the smoke
tent. A voltage regulator controls the
air inlet fan speed that is connected to the smoke generator. Within the smoke tent, the shelves can hold
the sown seeds on trays, seeds on screens, or can hold pans of water that can
be smoke-infused, depending on the germination method of choice. As with the heat germination, the temperature
and the smoke within the tent can be manipulated through the vents. We do not recommend exposing seeds to the
smoke in the tent for more than an hour.
If you are using water, the trays of water should remain in the smoke
tent for two hours.
Following smoke exposure
For each of the treatments,
the steps to take following smoke-exposure are listed below:
- Heat with smoke – If seeds are in seeding mix, place in
greenhouse until germination.
- Cooled smoke with seeds in seeding mix – place in greenhouse until germination.
- Cooled smoke with seeds on screens – You can sow seeds on desired seedbed
immediately following smoke treatment or store until needed.
- Smoke-infused water – Remove trays of smoke-infused water and pour
water into glass jars. Add seeds to
the jar of water. Put an electric air circulator/filter into the water and
circulate water for 24 hours. After
24 hours, you may either dry and store the seeds until they are needed or
sow the seeds directly, being sure to water daily for 6-10 days (see
figure 1 for an illustrated description).
Conclusion
With
increased urbanization, fire as a restoration tool is not always feasible. Smoke technology provides the ability to
incorporate the chemicals associated with fire in to a restoration when fire is
not possible. Numerous species
inhabiting fire-dependent ecosystems have evolved reproductive strategies to adapt
to factors associated with fire. The use of smoke has been shown to increase
the germination rate among some of these species. Seeds may be saturated with smoke either in
or out of soil, heated in smoke, or soaked in smoke-infused water. Because seeds
have different requirements the use of one or more of these techniques may be
necessary to stimulate germination.
A simple
smoke infusion system can be used to induce germination in dormant seeds. We designed a versatile apparatus to
accommodate all the methods of infusion mentioned above. With the use of this type of technology,
native plants with fire-related germination requirements may be more readily
used in restoration.
Literature Cited
Blank, R. R. and J. A.
Young. 1998. Heated substrate and smoke: influence on seed emergence and plant
growth. Journal of Range Management
51: 577-583.
Blommaert, K.L.J. 1972. Buchu seed germination. Journal
of South African Botany 38: 237-239.
Brown, N. A. 1993. Promotion
of germination of fynbos seeds by plant-derived smoke. New Phytology 123: 575-583.
Egerton-Warburton L.M. 1998.
A smoke-induced alternation of the sub-testa cuticle in seeds of the post-fire
recruiter Emmenanthe penduliflora Benth (Hydrophyllaceae). Journal of Experimental Botany 49:
1317-1327.
Coffey, M. 2003. Greening
Jager, A.K.; A. Strydom; J.
Van Staden. 1996. The effect of ethylene, octanoic acid and a plant-derived
smoke extract on the germination of light-sensitive lettuce seeds. Plant Growth Regulation 19: 197-201.
Jeffrey, D.J.; P.M. Holmes;
A.G. Rebelo. 1988. Effects of dry heat on seed germination in selected
indigenous and alien legume species in South Africa. South African Journal of Botany 54:
28-34.
Keeley, J.E. 1998.
Smoke-induced seed germination of California chaparral.
Ecology. October.
Morris, E. C. 2000.
Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and
scarification. Australian Journal of
Botany 48: 179-189.
Read, T. R., S. M. Bellairs,
D. R. Mulligan and D. Lamb. 2000. Smoke and heat effects on soil seed bank
germination for the re-establishment of a native forest community in New South
Wales. Austral Ecology 254: 48-57.
Regen 2000. 2003. Accessed
on 2 June 2003. http://www.tecnica.com.au/regen%20home.html
Roche, S., K.W. Dixon, and J.S.
Pate. 1997. Seed ageing and smoke: partner cues in the amelioration of seed
dormancy in selected Australian native species. Australian Journal of Botany 45: 783-815.
Roche, S.; J. Koch; K.W.
Dixon. 1997. Smoke-enhanced seed
germination for mine ehability in the south-west of Western Australia. Restoration Ecology 5: 191-203.
Tieu, A., K.A. Dixon, K.
Sivasithamparam. 1999. Germination of four species of native western Australian
plants using plant-derived smoke. Australian
Journal of Botany 47: 207-219.
Van Staden, J.; N.A.C.
Brown; A.K. Jager; and T.A. Johnson.
2000. Smoke as a germination
cue. Plant Species Biology 15:
167-178.