Lab Director: Dr. David Marcinek
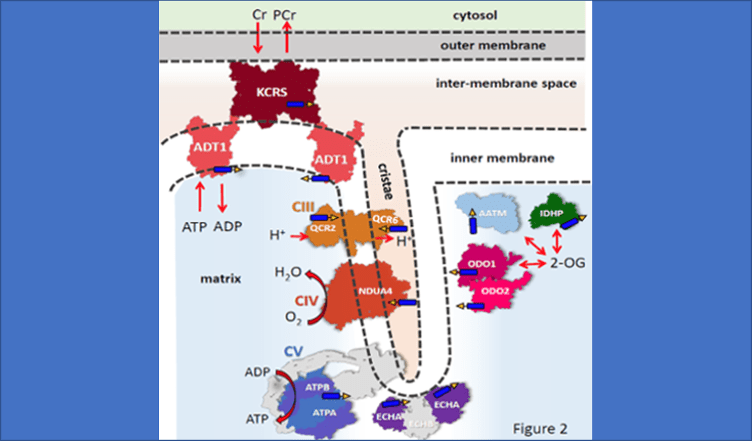
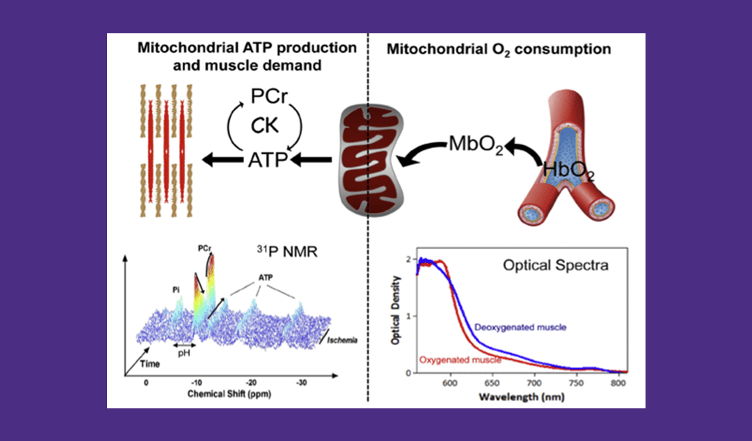
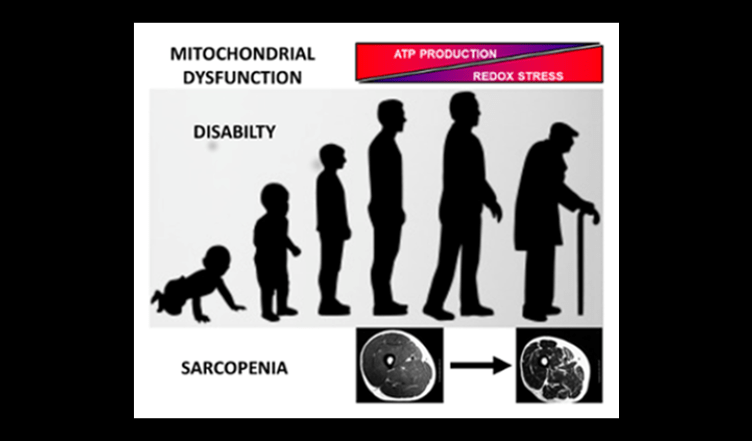
Work in the Translational Bioenergetics Laboratory (TBL) spans the range from molecular mechanisms of disease to testing targeted interventions in clinical trials. The overriding theme of research in the TBL is the interaction between mitochondria and cell stress and its effect on the pathology of chronic disease and aging. Much of our work focuses on skeletal muscle and mobility in aging, but given the broad impact of mitochondria our interests span multiple systems and diseases. Most people learn about mitochondria as kidney bean shaped structures that function as the “Powerhouse of the Cell” by generating chemical energy in the form of ATP. However, mitochondria are actually structurally and functionally dynamic organelles that sit at the nexus between cell energetics, redox biology, and cell signaling. As a result, mitochondria play an important role in the pathology associated with aging and many diseases, including some not typically associated with mitochondria like muscular dystrophies and chronic kidney disease. Our research indicates that even in cases where mitochondria are not the primary cause of disease, they can act to amplify the pathology and be important contributors to the decline in quality of life.
| Understanding the molecular changes in the mitochondrial that lead to changes to in vivo metabolism and contribute to disease. | Developing a pre-clinical to clinical pipeline to test new mitochondrial targeted interventions to improve healthspan. |
 |
 |
To support research into mitochondrial targeted interventions to improve healthspan:

Translational Bioenergetics Lab February 2024