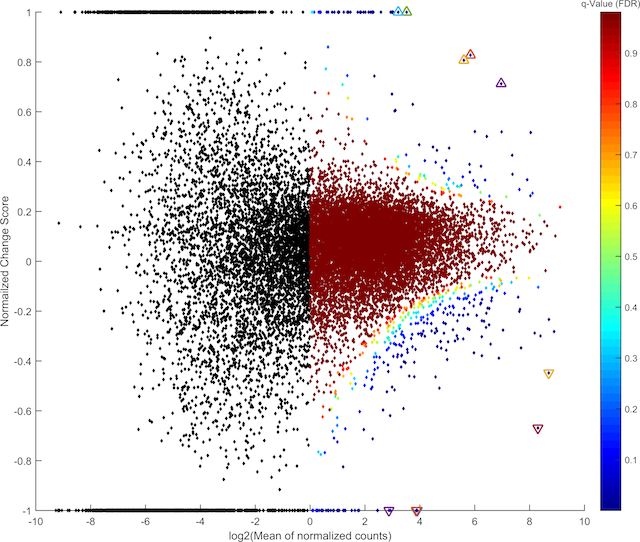
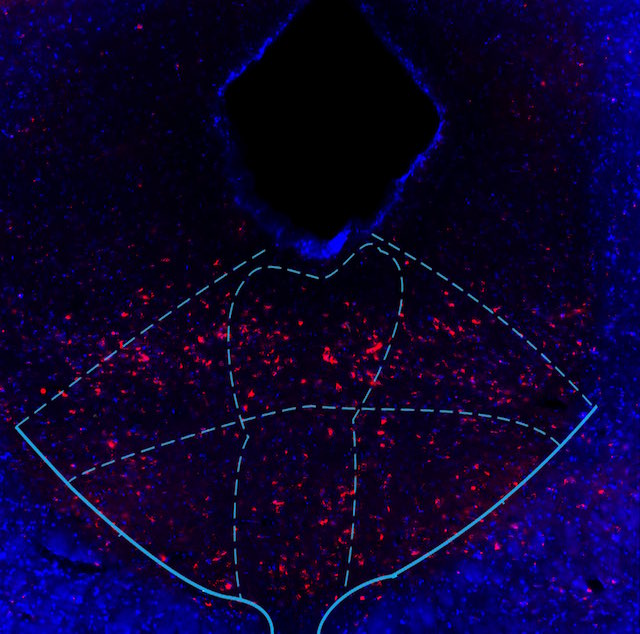
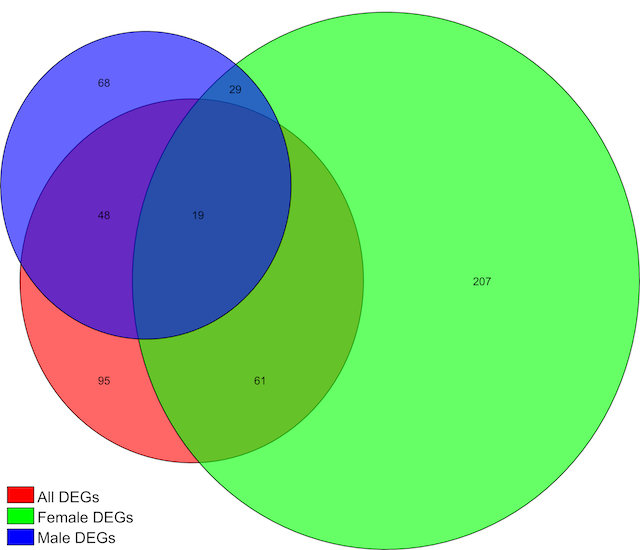
Given that the serotonin system plays a key role in stress responses and the pharmacological treatment of stress disorders, we presume that the adaptations associated with vulnerability and resilience are represented in cellular and molecular plasticity in the raphe neurons of individuals following exposure to stress, especially in pathways that are involved in signal transduction. This project will use mouse behavioral and molecular strategies to investigate these adaptations and to ask how resilience might be promoted while vulnerability might be prevented or reversed. Aim 1 will examine how modulating signal transduction pathways within serotonergic neurons might alter the behavioral phenotype of animals exposed to repeated social defeat stress. Our core methods will involve the expression of transgenes selectively in serotonergic neurons by using Cre-dependent viral vectors in mice that express Cre only in serotonergic neurons (ePet1-Cre mice). Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) that activate Gi or Gs signaling pathways prior to each session of repeated social defeat stress will allow for the examination how these manipulations alter stress responsiveness afterwards, using behavioral assays that assess different domains of depression-like and anxiety-like behavior. This Aim will provide new mechanistic and therapeutic clues regarding how plasticity in the serotonin system confers vulnerability or resilience to stress. Aim 2 will evaluate whether DREADD-mediated inhibition of serotonergic neurons in the interval after repeated social defeat stress can reverse the vulnerability to subsequent stressors, using corticosterone responses to restraint and immobility in the forced swim test to assess different domains of stress reactivity. Aim 3 will explore these behavioral stress models using a recently developed method to selectively interrogate the translation of mRNA in cells of interest, by expressing “RiboTag” only in the serotonergic neurons of ePET1-Cre mice. RiboTag is an epitope-tagged ribosomal protein that allows for antibody-mediated pull down only of polysomes from target cells derived from a complex cell homogenate (in this case a fresh midbrain tissue punch). Using this method, we expect to obtain ~50-fold enrichment of mRNA from serotonergic neurons compared to RNA extracted from the input sample. This will allow us to perform RNA-seq to investigate the totality of actively translated mRNA at key points during and after stress exposure. Efforts will be focused on mRNAs associated with excitability, signal transduction pathways, and cytokine responses, and use pathway analysis to identify co-regulation of mRNA translation in response to stress; this is intended to provide new mechanistic information and new targets for therapeutic development.
Publications
There are currently no publications associated with this project. Check back later for updates.