Cytochrome P450 3A4 (CYP3A4) is the major drug-metabolizing enzyme in humans. Because it has evolved to promiscuously oxidize a wide variety of xenobiotic compounds, CYP3A4 possesses a large and conformationally dynamic active site. The CYP3A4 active site can simultaneously accomodate multiple substrate and effector molecules, which leads to allosteric (non-hyperbolic) kinetic behavior — the binding of one or more effector molecules changes how substrate molecules bind to CYP3A4 or are metabolized.
Allosterism can be homotropic, where a single chemical species acts as both substrate and effector, or heterotropic, where one species affects the binding and/or turnover of another. Depending on the combination of substrate and effector, CYP3A4 can display either positive cooperativity (binding and/or turnover are enhanced by multiple binding events) and negative cooperativity (binding/turnover are diminished).
Heterotropic allosteric behavior is a major cause of drug-drug interactions, where the administration of one drug alters the metabolism of another. Drug-drug interactions are of therapeutic importance because they can lead to increased toxicity, deleterious side-effects and decreased efficacy of prescribed medications. This makes it important to understand the causes of CYP3A4 allosterism and therefore of many adverse drug-drug interactions.
We use a variety of biophysical techniques including fluorescence and absorbance spectroscopy, NMR, electron paramagnetic resonance (EPR) and surface plasmon resonance (SPR) to study how various ligands bind to CYP3A4, so as to explain observed allosterism in catalytic behavior.
We have extensively studied the binding of testosterone (TST) and a-naphthoflavone (aNF), which are known to exhibit both heterotropic and homotropic cooperativity, to better understand the relationship between cooperativity and metabolism. Using UV-vis and EPR spectroscopy, the individual binding events of these drugs to CYP3A4 were deconvoluted and represented in a simplified thermodynamic model. This thermodynamic model is consistent with the X-ray crystal structure of CYP3A4 and the known metabolism of these drugs.
In addition to NMR and SPR, fluorescence is a useful non-destructive method for obtaining information on ligand-enzyme dynamics that may reveal details regarding the mechanistic basis for cooperativity in CYP3A4. Recently, we have used the fluorescent compounds TNS (2(p-toluidino)naphthalene-6-sulfonate) and Nile Red as probes of conformational change and heterotropic ligand binding events in CYP3A4. Time-resolved frequency-domain measurement of TNS fluorescence lifetimes indicates a testosterone (TST)-dependent decrease in the excited state lifetime of TNS, concomitant with a decrease in steady state fluorescence intensity. In contrast, the substrate erythromycin (ERY) had no effect on TNS lifetime, while it decreased the steady state intensity. Together, the results suggest that TNS binds in the active site of CYP3A4, while the first equivalent of TST binds at a distant allosteric effector site.
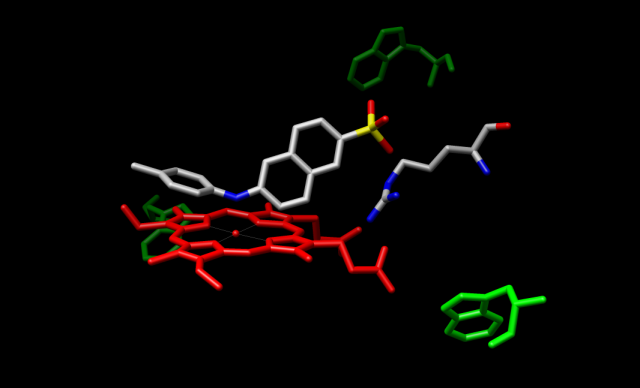
Model of TNS bound in the CYP3A4 active site
In addition, we have demonstrated that in an optical titration with 3A4, Nile Red induces a concentration dependent heme spin state shift characteristic of a type I ligand, with an approximate KD of 3 μM. Also, Nile Red is efficiently metabolized by 3A4, producing both N-monoethyl and N-desethyl metabolites, with a KM of ~10 μM. In fluorescence titration experiments with CYP3A4, Nile Red demonstrated a clear concentration dependent increase in fluorescence intensity reaching saturation at a ligand concentration of 12 μM. When fit to a single site binding model, the data indicate that the KD for the Nile Red-CYP3A4 complex is approximately 9.8 μM. Furthermore, the fluorescence emissions spectra are red-shifted, indicating that the Nile Red ligand is in the "twisted" state (nitrogen substituents perpendicular to the plane of the ring system) when bound to 3A4. Future experiments will include time-resolved fluorescence anisotropy studies to determine relative rotation rates of CYP3A4-bound Nile Red in the presence and absence of a variety of known 3A4 effectors.