4-Hydroxynonenal (HNE) is a toxic end product of lipid peroxidation that has been implicated in pathological states such as neurodegenerative diseases and cancer. Human glutathione S-transferase A4-4 (hGSTA4-4) has been shown to catalyze the 1,4-addition reaction of GSH with HNE. Once HNE is metabolized, the resulting conjugate (GSHNE) can be specifically transported out of the cell by the ATP-dependent transporter, RLIP76.
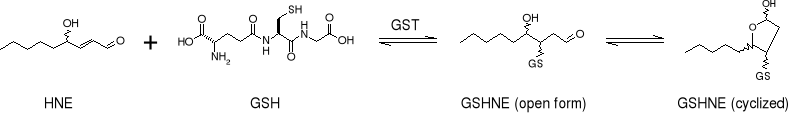
Given the innate chirality of proteins we hypothesize that the conjugation could be stereospecific and subsequently influence transport. LC/ESI-MS analyses using the racemic- and enantioisomeric-HNE substrates indicate that hGSTA4-4 catalysis exhibits stereospecificity with regard to the nucleophilic attack by the GSH sulfur on C3 of HNE (C4 of GSHNE). Furthermore, results from RLIP76 transport studies demonstrate a matching stereoselectivity with regard to the transport of the different GSHNE diastereomers.
A combination of the results from 1D-WATERGATE, 2D-COSY, and 2D-ROESY NMR techniques are currently being used to assign the proton chemical shifts and model the three dimensional conformations of the different GSHNE diastereomers.
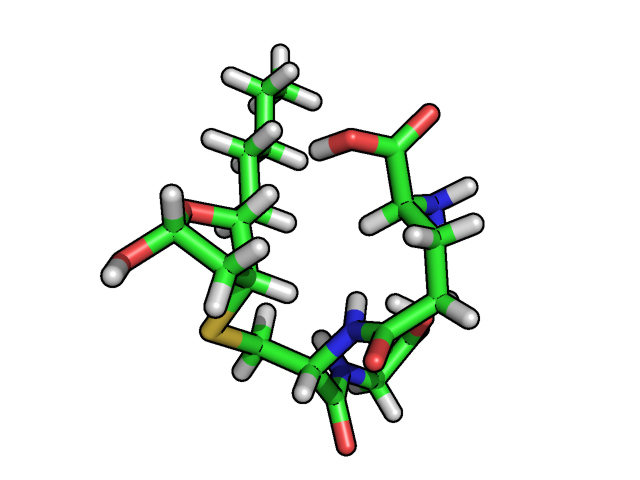
Model of a GSHNE diastereomer based on NMR restraints