I.7: Translating Between Velocity Equations and Binding Equations
So far we have devoted all of our attention to modeling turnover kinetics. Of course, many experiments focus not on catalysis but on equilibrium binding, using absorbance or fluorescence changes induced by substrate binding as observable signals. The strategies we used to derive velocity equations for kinetic models are easily adapted to derive binding equations for equilibrium models. Basically, all KMs are replaced by KDs and all Vmax values are replaced by appropriate scaling factors for the observed signals.
For example, consider a two-site sequential equilibrium binding model:
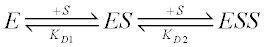
If the ES and ESS species have differential responses to the experimental technique used, they will each have a maximal response Bmax1 and Bmax2. Then the equilibrium binding equation (where B is the observed signal) is:
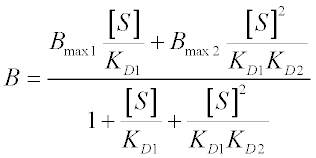
This translation can be done for any of the kinetic models we have covered previously.