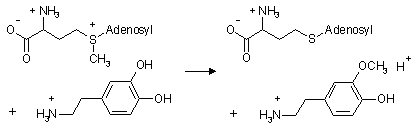|
Velocardiofacial Syndrome: Enzyme Stabilization
Therapy?
Nearly half the US population carries a
polymorphism of the gene for catechol-O-methyltransferase (COMT) that
results in substitution of methionine for valine at position 108 of the
soluble form of the protein (s-COMT) and position 158 of the
membrane-bound form (mb-COMT). The Met variant of s-COMT loses activity
rapidly at 37o, while the Val variant is stable. Individuals who are
homozygous for the 108/158Met allele are at increased risk for several
neuropsychiatric conditions. The instability of the Met variant appears
to be a particularly serious problem in patients with velocardiofacial
syndrome, who are missing the COMT gene entirely on one copy of chromosome
22. We propose to investigate why the Met variant is unstable, and to
explore ways of stabilizing it. To determine whether the thermal
inactivation reflects global unfolding or more subtle conformational
changes, we will correlate the enzyme activity of 108Met and 108Val s-COMT
with a panel of spectroscopic properties that report on protein
conformation as functions of temperature, time, pH, and the substrate
S-adenosylmethionine. Regions of the protein that unfold first or that
dominate the rate-determining step of unfolding are potential targets for
enzyme-stabilizing small molecules. We will identify these regions by
using mass spectroscopy to measure the rates of deuterium exchange of
amide hydrogens, by molecular-dynamics simulations of the thermal
unfolding pathway, and by exploiting fluorescence correlation spectroscopy
to examine the dynamics of structural fluctuations near the active site.
Similar studies will be conducted on mb-COMT, which probably is the
physiologically important form of COMT in the brain. We will select the
most promising potential drug-binding sites based on the spectroscopic
studies, deuterium exchange maps, and molecular-dynamics simulations, and
we will then carry out iterative cycles of computational docking protocols
and experimental assays to discover and refine molecules that stabilize
108/158Met COMT. Although beyond the scope of this proposal, lead
compounds obtained from these searches can be tested in an existing mouse
model of COMT deficiency.
Velocardiofacial Syndrome - Biological
Significance
Velocardiofacial syndrome (VCFS) is now
recognized to be the most common contiguous-gene deletion syndrome in
humans, having an estimated prevalence of 1:4000 births (A contiguous-gene
deletion syndrome refers to the loss of several contiguous genes in a
particular chromosome). The majority of individuals with VCFS are missing
a common stretch of 3 Mb on the short arm of chromosome 22. In most cases
the disorder is not inherited, but rather appears to arise de novo from an
error in homologous recombination. The error probably is mediated by
duplicated sequences ("low-copy repeats") that flank the deleted
region..
VCFS has a highly variable clinical phenotype, including more than 180
physical finding. DiGeorge originally described a condition of
immunological insufficiency associated with developmental problems of the
heart, vascular system and thymus. Shprintzen et al. independently
described a syndrome of anomalies of the soft palate (velum) and face in
addition to the heart. Anatomic anomalies of the brain have been found,
and mild neurological impairment of cognition, attention, concentration
and social-emotional function is common. Many individuals with VCFS
develop a characteristic spectrum of severe neuropsychiatric
manifestations that include pronounced separation anxiety, night terrors,
and wide mood swings in early childhood, obsessive-compulsive disorder in
later childhood, and a combination of an
"ultra-ultra-rapid-cycling" bipolar disorder with characteristic
"affective storms," anxiety, perseverative thoughts and
hallucinations in adolescence. Some investigators consider the most common
psychiatric symptoms to be consistent with schizophrenia or
schizoaffective disorder, while others have emphasized features
characteristic of bipolar and attention-deficit disorders. The
neuropsychiatric manifestations of velocardiofacial syndrome are
remarkably resistant to most neuropharmacological treatments, and have a
devastating impact on the quality of life for many patients.
The commonly deleted region of chromosome 22q11 spans at least 30
genes, one of which is the gene for catechol-O-methyl transferase (COMT).
COMT inactivates the neurotransmitter dopamine by transferring a methyl
group from S-adenosyl-methionine (SAM) to a phenolic group of the catechol
ring:
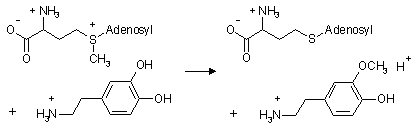
It acts similarly on epinephrine and norepinephrine. COMT also
participates in the catabolism of catecholestrogens and of catechol drugs
used for treatment of Parkinson disease, hypertension and asthma. In the
brain, a deficiency of COMT could result in higher concentrations of
dopamine, which could plausibly contribute to the neuropsychiatric
manifestations of velocardiofacial syndrome. Other genes in the deleted
region have been implicated in developmental aspects of VCFS and could
contribute to the neuropsychiatric symptoms, but none of the other genes
that have been identified has as clear a connection to neurochemical
function.
Human COMT occurs as both a soluble protein with 216
residues (s-COMT, 24.4 kDa) and a membrane-bound protein with an
N-terminal extension of 50 residues (mb-COMT, 30.0 kDa). The two proteins
are coded by a single gene that has two start sites for transcription, but
little is known about the factors that determine the relative amounts of
the two forms in a given tissue. The soluble form of the protein
predominates in most tissues except for brain and adrenal medulla, where
mb-COMT is the major species. In the brain, the enzyme is found mainly in
postsynaptic neurons and in glial cells near synapses and capillary walls.
The membrane-bound form seems likely to play the main role in dopamine
catabolism, because its Km for dopamine is about ten-time smaller than the
Km of the soluble form.
The crystal structure of rat s-COMT has been
determined at a resolution of 2 Å. Human s-COMT has not been
crystallized, but probably has a 3-dimensional structure similar to that
of the rat enzyme because the amino acid sequences are about 80%
identical. No structure is available for mb-COMT; however, a hydropathy
plot indicates that its additional N-terminal domain probably has a single
transmembrane -helix.
A common polymorphism in the COMT gene results
in substitution of methionine for valine at amino acid 108 of the soluble
protein and position 158 of the membrane-bound enzyme. Approximately 25%
of the US population is homozygous for the 108/158Met allele. The Met
variant has markedly lower thermostability at physiological temperatures,
and individuals who are homozygous for this allele have a 3- to 4-fold
lower level of enzyme activity in their erythrocytes. Purified 108Met
human s-COMT was found to lose about 80% of its activity in 30 min at 37o,
while the activity of the 108Val enzyme was essentially unchanged. In
patients with velocardiofacial syndrome, having the 108/158Met allele on
the non-deleted chromosome could compound the effects of loss of the gene
from the other copy of chromosome 22. In line with this reasoning,
hemizygosity for the 108/158Met variant is strongly associated with some
of the most severe neuropsychiatric manifestations of VCFS. In the general
population, homozygosity for 108/158Met is associated with increased risk
for obsessive-compulsive disorder in males, "ultra-rapid
cycling" bipolar disorder, late-onset alcoholism and depressive
disorder, and with aggressive, homicidal and suicidal behavior in
schizophrenics. Although the 108/158Met variant of COMT is clearly not the
cause of schizophrenia or bipolar disorder it evidently can intensify
these disorders. In a mouse model, disruption of the COMT gene leads to
increased aggression and behavioral changes indicative of increased
anxiety. The less stable form of COMT also has been associated with
increased risk for breast cancer in humans, although other investigators
have found no such association.
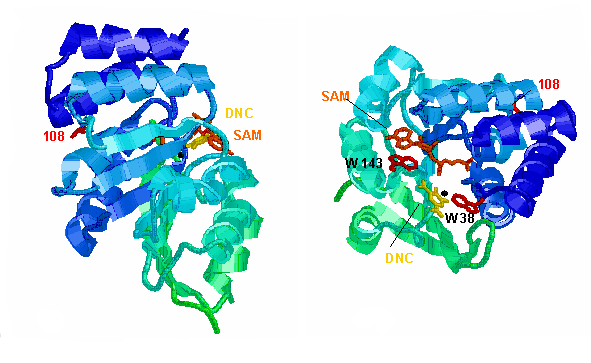
Above are cartoon representations of the crystal structure
of rat s-COMT from two viewpoints. The side chain of residue 108 (leucine
in the rat) is shown in red. SAM, dinitrocatechol (DNC, a tightly binding
inhibitor) and Mg2+ are in orange, yellow and black, respectively. Shades
of blue and green indicate secondary structural elements ( -helices,
-sheets and loops). The coordinates are from Brookhaven file 1VID.PDB.
|