 |
|
|
Fall 2016 Isotope Analysis with Thiobacillus DenitrificansBy Matthew Koehler 
It was Autumn 2015 when I flew northeast from Seattle with a final destination of St. Andrews, Scotland. I lived there for three months while I completed my research rotation with Dr. Aubrey Zerkle and Dr. Mark Claire (UWAB alum). I met Aubrey and Mark at the 2014 annual American Geophysical Union conference in San Francisco; the largest gathering of geologists, Earth scientists, educators, and policy makers in the world with upwards of 24,000 attendants. I was presenting a poster on some preliminary results from that year’s research, and they both stopped by my poster to chat about our academic commonalities, which primarily revolve around the study of nitrogen cycling in Earth’s ancient oceans. A few months later I contacted them to express interest in doing a research rotation at St. Andrews. The rotation project for the UWAB program has to be sufficiently distanced from one’s primary research, but can still be related in some ways (this is rather a grey area decided by the powers that be). My primary research investigates the evolution of the ancient nitrogen cycle, which can provide insight into how life and the environment have changed together through time. Much like how dinosaurs left behind bones that can tell us how and when certain dinosaurs lived, ancient microorganisms left behind traces sometimes referred to as atomic fossils that contain information about the conditions under which certain microorganisms were living. Within the framework of studying nitrogen cycling, these atomic fossils are the relative abundances (or isotopic ratio) of two nitrogen isotopes preserved in the rock record; 14N with 7 protons and 7 neutrons, and 15N with 7 protons and 8 neutrons. In a practical sense, I collect sedimentary rocks that were deposited in the ancient ocean anytime from 400 million years ago to 3 billion years ago. I then measure the abundance of these nitrogen isotopes that are derived from ancient microbial biomass, and based on their relative abundances I am able to interpret the results as if it were a cohesive fossil; roughly determining which nitrogen metabolisms made up the ecology at the time these sediments were being deposited. This is possible because, as observed in the modern nitrogen cycle, each step in the biologically mediated nitrogen cycle has an effect on the relative abundance of the two nitrogen isotopes (figure 1). Indeed, an ecology dominated by nitrogen fixers (microorganisms that take nitrogen from atmospheric N2) can leave a uniquely different nitrogen isotope signal in the sediment record compared to an ecology dominated by nitrifiers, denitrifiers, and microorganisms that take up nitrate. 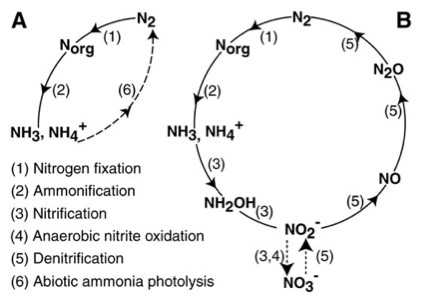 Figure 1: A schematic of two possible nitrogen cycle regimes: (A) Anaerobic dominated by nitrogen fixation, and (B) Aerobic with nitrification and denitrification. Steps 1-5 are biologically mediated, and affect the nitrogen isotope ratios in different ways As hinted at above, the interpretation of atomic fossils relies on our understanding of how modern biology affects these isotope abundances, and this is true for any bio-isotopic system. In 1939, American physicist Alfred Nier and his colleague Earl Gulbranson first discovered that the relative abundances of carbon isotopes (13C/12C) varied in nature, noting that the isotopic composition of carbonate was different from that of plant matter. It was not long before microbiologists started looking for variations within the biosphere, addressing questions like: Does the biomass of photosynthetic bacteria have a similar carbon isotope ratio compared to the biomass of methanotrophic bacteria? Dozens and dozens of microbiological experiments followed, answering questions like the one above, not only in regards to carbon isotopes, but also nitrogen, sulfur, and many others. The result has been a growing understanding of how modern microbes affect different isotopic systems. So in a very broad sense, the whole field of isotope biogeochemistry can be split into two practices: The first is growing modern microorganisms in the laboratory, determining how different metabolisms affect the isotopic ratios of different elements. The second is applying what we learn from the modern laboratory experiments to the geologic record. The latter is what I do for my primary research at the UW, the former is what I did for my research rotation at St. Andrews. This is where things get a little more detailed. The microbe we used in my laboratory experiments and St. Andrews is called Thiobacillus Denitrificans (TD). TD is what is called a sulfur-oxidizing bacteria, but unlike other sulfur-oxidizing bacteria TD grows under anaerobic conditions; it does not use oxygen to oxidize sulfur. Instead, TD uses oxidized nitrogen species like nitrate and nitrite to oxidize reduced sulfur species. The specific reaction in these experiments is as follows: Why this microbe, and why this reaction? Both nitrogen and sulfur isotopes are used in the geologic record to interpret past microbial ecology and environmental conditions. There are times in Earth’s history when this metabolism may have had a larger ecologic role, and thus may have influenced the isotopic record of either/both nitrogen and sulfur. Knowing how this metabolism affects the isotope ratios of nitrogen and sulfur isotopes may then be important in interpreting the geologic record. During this research rotation, I learned everything I know about culturing anaerobic microbes from Aubrey. The first step was creating the growth media – the aqueous environment in which our microbes would grow in. Once we nailed down the growth media, we inoculated our first generation cultures. While the first-gen cultures were growing, Mark showed me how to use his ion chromatograph, an instrument we would use to monitor nutrient changes in the media as our bacteria grew (as the reaction above suggests, as the bacteria grow, thiosulfate and nitrate should be decreasing in the media, and sulfate and nitrogen gas should be increasing). We inoculated the second generation of microbes from the first, and subsampled the media at set time intervals to monitor nutrient changes as the bacteria grew. We did this to generate growth curves for these cultures, which provide information on the rate of growth through time (figure 2). Once growth curves were defined for our cultures, we inoculated new cultures. From these new cultures, we subsampled the media at time intervals along our already generated growth curves (more refined time-steps). These subsamples were split into two groups, one was measured on the ion chromatograph again, and the other subsamples were brought back to UW to await both sulfate and nitrate isotopic analyses. 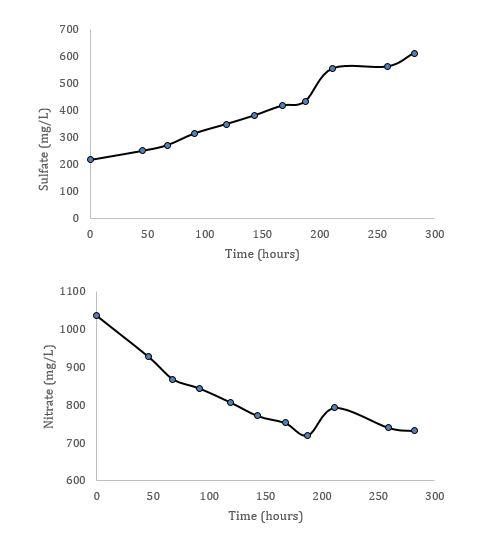 Figure 2: Example growth curves from one of our trials “Alpha”. Notice how sulfate increases and nitrate decreases, consistent with the given reaction for this metabolism. Subsamples were taken at each dot, and await isotopic analyses. As thiosulfate goes to sulfate and as nitrate goes to nitrogen gas, we should be able to track the changes of the isotope ratios in the nitrate and sulfate as the bacteria grow. This will give us an idea of how this metabolism affects both isotopic systems. The majority of biology-mediated reactions follow the expression life is lazy, which refers to the product being more enriched in the light isotope over the heavy when compared to the reactant (life selects the lighter isotope). Does this apply to our metabolism of interest, and to what degree, are questions we look to address with our experiments. It is possible that the isotopic effects for both sulfur and nitrogen from this reaction are similar to other, more widespread metabolisms. This would make it difficult to interpret this metabolism in the geologic record. There is, however, always something to be learned by doing these microbiological-isotope experiments on understudied metabolisms that can benefit the field of isotope biogeochemistry. Currently, the subsamples for isotope analyses are waiting for analysis in the refrigerator on the 3rd floor of Johnson Hall at the UW. When I get the time, I look forward to completing these analyses and viewing the results. I am very grateful for the hospitality and instruction provided by Dr. Aubrey Zerkle and Dr. Mark Claire, as well as the other faculty and students at St. Andrews who were incredibly helpful and kind. I feel fortunate to have completed my UWAB research rotation in a scientific community filled with such experts, and also in a place that is filled with stunning beauty in the right light (i.e. unobstructed by torrential downpours). I would be remiss if I did not thank Dr. Garath Izon for sharing his home with me for my time in St. Andrews, and also Dr. Tony Prave for taking me to the field to collect some sought-after samples!  A snapshot of part of St. Andrews after a lovely day. 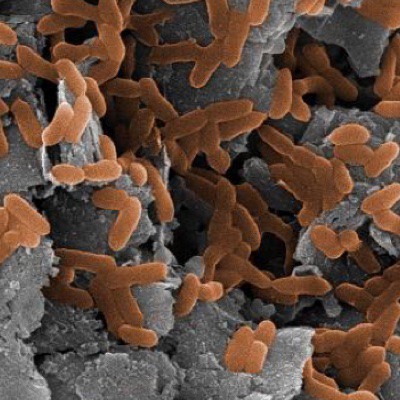 Image of Thiobacillus Denitrificans. Taken from Coral Wonders.  Anaerobic chamber similar to the one I used at St. Andrews to make the growth media.  An Ion Chromatograph similar to the one I used at St. Andrews to monitor nutrient changes in the growth media through time.  A photo of the cottage I stayed at during my time at St. Andrews, located on a farm just outside of town. Also shown is the commuter bike Aubrey and Mark lent me! About the author: Matt Koehler is a third-year Dual-Title ESS and Astrobiology PhD student and a member of the IsoLab at the University of Washington. |
|
