B4T R.A.T
This tool provides a format for researchers to systematically identify and control hazards to reduce risk of injuries and incidents. Conduct a risk assessment prior to conducting an experiment for the first time and review the Lab R.A.T. Guidelines document for further details.
The risk assessment process involves rating the risk of the experiment from “low” to “unacceptable” risk. Consult with your PI/supervisor and EH&S if your risk rating is “high” or “unacceptable” to redesign the experiment and/or implement additional controls to reduce risk.
Phase 1: Explore
Identify your research question and approach. What question are you trying to answer? What are you trying to measure or learn? What is your hypothesis? What approach or method will you use to answer your question? Are there alternative approaches?
Phase 2: Plan
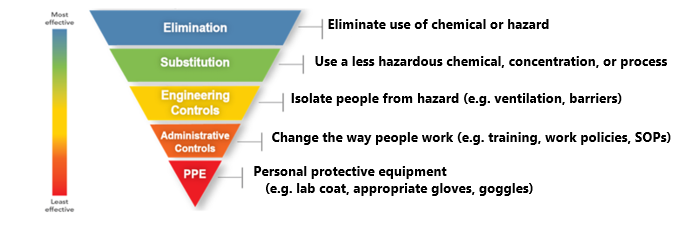
Outline the Procedure. List the steps or tasks for your procedure and the hazard/potential consequences of each. Include set-up and clean-up steps or tasks. Define the hazard controls to minimize the risk of each step using the hierarchy of controls starting with the most effective (i.e., elimination, substitution, engineering controls, administrative controls, and personal protective equipment). List the hazard control measure you would use for each step or task (e.g., run at a micro scale, work in a fume hood, wear face shield and goggles).
1 For guidance on selection of Personal Protective Equipment (PPE), use EH&S PPE Hazard Assessment Tool.
2 For guidance on selection of chemical-resistant gloves, see EH&S Website.
A hierarchy of controls should be applied starting with the most effective controls (i.e., elimination and substitution) at the top of the graphic and moving down. While personal protective equipment (PPE) should always be used, it should be considered the last line of defense from potential hazards.
Phase 3: Challenge
Question your methods. What have you missed and who can advise you? Challenge your hazard control measures by asking “What if…?” questions. “What if” questions should challenge you to find the gaps in your knowledge or logic. Include possible accident scenarios. Factors to consider are human error, equipment failures, and deviations from the planned/expected parameters (e.g., temperature, pressure, time, flow rate, and scale/concentration). Update your plan to include any new controls required to address these possibilities.
WHAT IF ANALYSIS
Examples:
What if…there is a loss of cooling? …valves/stopcocks are left open/closed? …there is unexpected over-pressurization? …a spill occurs? …the laser is misaligned? …weather conditions change?
Then… there may be a runaway reaction. …there may be an unexpected splash potential. …the reaction vessel may fail. …there may be a dermal exposure. …there may be an eye injury. …routes may be inaccessible.
Assign a risk rating to the experiment.
Based on your procedure outline and the what if analysis, determine the risk rating for the experiment or procedure.
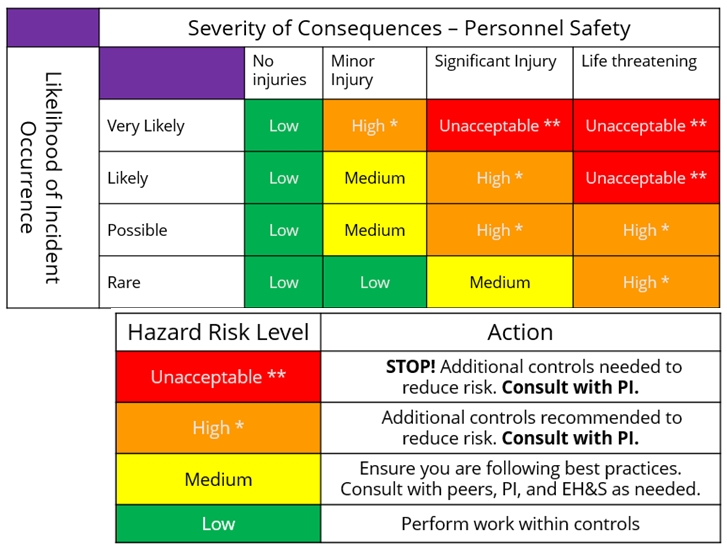
Revise plan if the risk rating is too high. Are these risks acceptable? Use this table to determine the action to take based on the risk rating. What are the highest risk steps? What more can you do to control the risks? Return to planning and use the hierarchy of controls to design a safer experiment.
NOTE: **Unacceptable risk-rated experiments should not proceed. Introduce further controls to reduce risk. Contact EH&S (206.221.2339) for recommendations and best practices.
Phase 4: Assess
Perform a trial run. How you can test your experimental design? Can you do a dry run of the procedure without hazardous chemicals/reagents/gases to familiarize yourself with equipment and demonstrate your ability to manipulate the experimental apparatus? Can you run the procedure with a less hazardous material? Can you test your experimental design at a smaller scale? If your procedure requires multiple people, would a tabletop exercise be useful?
Perform and evaluate. Run your procedure using the appropriate controls you’ve identified. Evaluate controls and hazards as you work. Critique the controls and process you used by answering the following questions. If changes to controls are needed, update your risk assessment tool and re-evaluate any time you revise your process (e.g. changes in scale, reagent, equipment, or conditions that might increase the hazard/risk). Share your assessment with your PI/colleagues for the next iteration of the experiment.
