 |
David Veesler
Hans Neurath Endowed Chair in Biochemistry
Professor of Biochemistry
Investigator, HHMI
Ph.D. 2010, Aix Marseille University, Marseille, France
|
Research
Macromolecular machines occur ubiquitously in nature where they achieve a broad spectrum of biological functions. These nano-assemblies constitute the essence of life as proteins and nucleic acids often accomplish their tasks when included in a multi-protein complex, either stable or transient. As a consequence, macromolecular complexes are of significant medical interest due to the fact that perturbations of protein/protein or protein/nucleic acid interactions can lead to a number of diseases or alternatively be used for drug discovery. A detailed knowledge of the structure and function of these molecular assemblies is therefore key to understanding basic biological processes and expedite advances in medicine.
Our main research interest is to tackle the structure of macromolecular machines of biological interest to understand the mechanisms underlying their functions. We use a multi-disciplinary approach involving cryo-electron microscopy and X-ray crystallography complemented by various biochemical and biophysical techniques to obtain multi-scale data ranging from atom to whole-cell.
Cryo-electron microscopy is an increasingly important technique in structural biology, which enables the study of biological macromolecules in a near-native environment. This method is undergoing a technical revolution due to the recent developments of direct detectors, which significantly enhance its potential and pave the way toward the routine achievement of near-atomic resolution reconstructions for samples of moderate size and symmetry in the near future. Cryo-electron microscopy also enables studying samples featuring marked conformational flexibility and/or compositional heterogeneity and that are not amenable to structural characterization using other techniques. It is thus possible to obtain various snapshots of macromolecular machines yielding insights into their mechanisms of action.
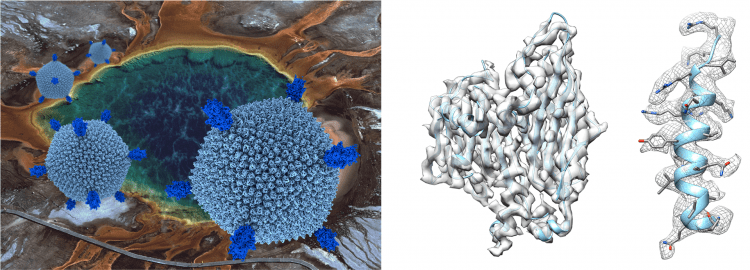
The secret of life in boiling acids.
(Left) Sulfolobus Turreted Icosahedral Virus infects the hyperthermo-acidophilic archeon Sulfolobus solfataricus, which lives in acidic hot springs at Yellowstone National Park (temperature 80 °C and pH 3). The association of extreme environments with the physico-chemical conditions of the “primordial soup” fosters interest in Archaea and their viruses as their study may shed light onto the emergence of life as well as its evolution.
(Right) We determined the STIV structure at 3.9 Å resolution using single-particle cryo-electron microscopy. The three-dimensional reconstruction allowed identification of many structural proteins and tracing of their polypeptide chains due to well-resolved features in the density. The findings point to a mechanism of virion assembly through a complex coordination of interactions of the viral membrane and various protein components. Furthermore, the outcome of this study supported the hypothesis that STIV belongs to a lineage including Eukaryotic, Bacterial and Archaeal viruses deriving from a common viral ancestor present in the biosphere more than 3 billion years ago.
Reference: Veesler D, Thiam Seng N, Lawrence MC, Lok S, Johnson JE and Fu CY. Atomic structure of the 75mega Dalton extremophile Sulfolobus Turreted Icosahedral Virus determined by CryoEM and X-ray Crystallography. Proc Natl Acad Sci. (2013) 110(14):5504-9. |
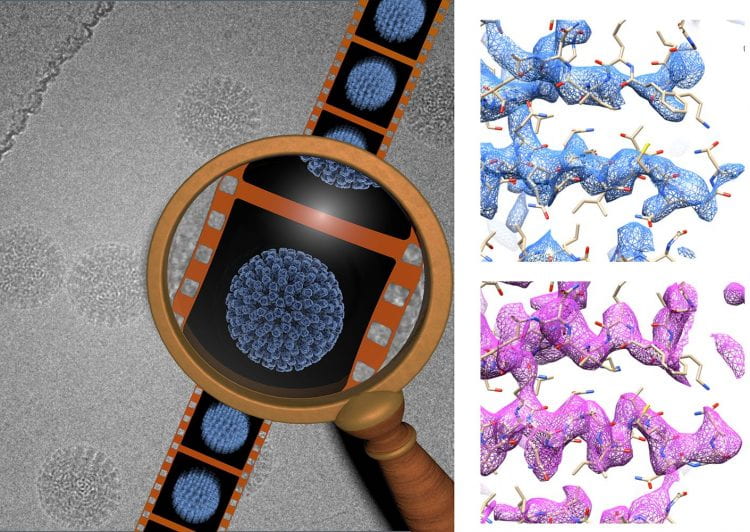
Near-atomic resolution cryo-electron microscopy using direct detectors.
(Left) A common problem in cryo-electron microscopy is the blurring in images due to beam-induced sample movement. Recording movies of ice-embedded samples using direct detectors during beam exposure allows tracking particle movement and aligning individual frames to reducing blurring.
(Right) The combination of the new generation of direct detection cameras along with this method accounting for beam-induced specimen motion significantly enhances the potential of cryo-electron microscopy to yield three-dimensional reconstructions at near-atomic resolution. This is illustrated by the improvement in map quality observed by comparing the density of the human rotavirus VP6 subunit before (4.9 Å resolution, blue) and after (4.4 Å resolution, pink) accounting for sample movement.
Reference: Campbell MG, Cheng A, Brilot AF, Moeller A, Lyumkis, D, Veesler D, Pan J, Harrison SC, Potter CS, Carragher B and Grigorieff N. Movies of ice-embedded particles enhance resolution in electron cryo-microscopy. Structure. (2012) 20:1-6 |
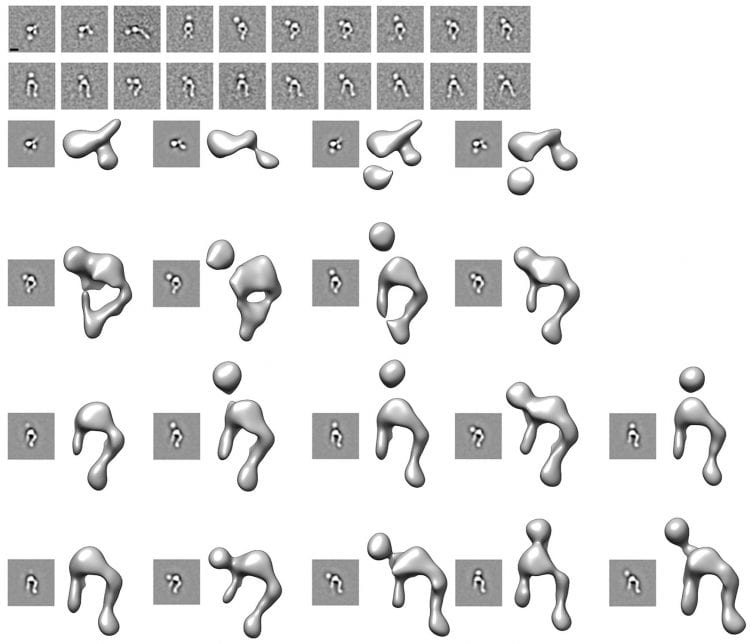
How a virus used for gene therapy enters cells?
(Top) We characterized the interactions between the human adenovirus 9 penton base subunit and αVβ3 integrin (the entry receptor) to understand the mechanisms underlying virus internalization and infection. The results are illustrated by the class-averages of the complex obtained by negative staining electron microscopy revealing the penton base binding at distinct sites on the integrin as well as the marked flexibility of the later.
(Bottom) Three-dimensional reconstructions of the complex confirmed that the adenovirus 9 penton base binds at various locations onto the integrin headpiece, suggesting a putative mechanism to enable integrin clustering and promote virus entry into the host cell. This work also showcases the potential of single particle electron microscopy to deal with conformationally flexible samples.
Reference: Veesler D, Cupelli K, Sthele T and Johnson JE. Single particle EM reveals plasticity of interactions between the adenovirus penton base and integrin αVβ3. Proc Natl Acad Sci. (2014) 111(24):8815-9. |
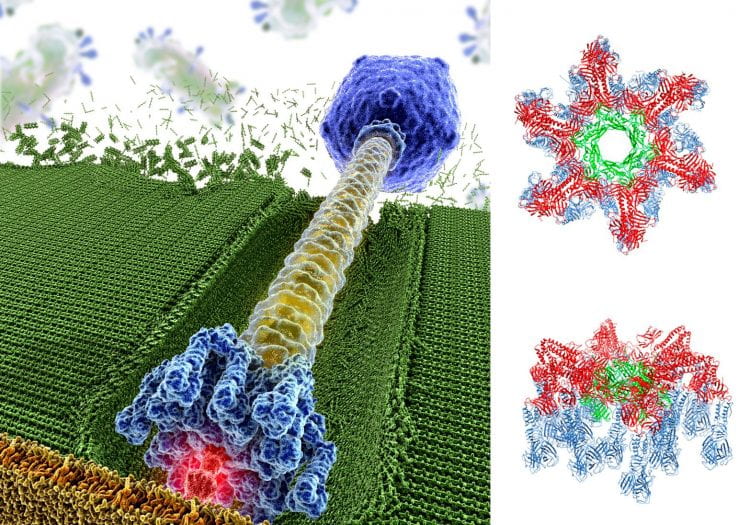
Crystal structure of a virus infection device.
(Left) Bacterial viruses are efficient nanomachines possessing a tail appendage used to recognize the host and ensure genome delivery via perforation of the host cell wall.
(Right) We solved the crystal structure of the 1.8 MDa host adsorption device of the phage TP901-1, which infects Lactococcus lactis (a bacteria widely used in the dairy industry). The structure determination of this 78-protein subunit complex constituted a “tour-de-force” that led to significant new insights about virus infection to help preventing phage “outbreaks” in an industrial context.
Reference: Veesler D, Spinelli S, Mahony J, Lichière J, Blangy S, Bricogne G, Legrand P, Ortiz-Lombardia M, Campanacci V, van Sinderen D and Cambillau C. Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc Natl Acad Sci. (2012) 109(23):8954-8. |
Publications:
Retrieving citations from PubMed, please stand by….





