Bloodstream Infections: Epidemiology & Risk Factors
Peripheral and central venous catheters are commonly used in health care delivery worldwide. These devices increase a patient’s risk for developing bloodstream infections (BSIs) and other local infections in and around the insertion site. In this module, you will learn about case definitions of BSIs due to catheter use, its epidemiology and the risk factors for acquiring BSIs.
Learning Objectives
By the end of this module you will be able to:
- define key terms and case definitions; and
- describe the epidemiology and risks for infections associated with the use of peripheral and central venous catheters.
Learning Activities Estimated time:
-
Walid’s Story (5 min)
Walid, a 56-year-old man, was admitted to the coronary care unit (CCU) after having a heart attack. While in the CCU, physicians inserted a central venous catheter (CVC) via his internal jugular vein. Three days later, Walid became febrile with a temperature of 38.5°C. Blood cultures, which were sent to the laboratory, came back positive for Enterococcus faecalis, a recognized invasive pathogen known to cause severe BSI. At the time, no other possible sources of infection have been identified.
-
Terms and Case Definitions (10 min)
Before we talk about how to prevent BSI, let us begin by looking at a few definitions. In this module, we present definitions as defined by the European Centre for Disease Prevention and Control (ECDC)1 and the United States Centers for Disease Control and Prevention (CDC).2
Bloodstream infections
There are two types of BSI. Primary infections are unrelated to an infection anywhere else in the body; they are instead potentially linked to the use of a vascular access device. In other words, the infection is caused by the use of a vascular device. Secondary infections are related to an infection at another place in the body (e.g., pneumonia, urinary tract infection or surgical site infection). If a blood culture is positive, you must always search for a source.
One positive blood culture for a recognized pathogen (note: this is different than a skin contaminant) Patient has at least one of the following signs or symptoms: fever (>38°C), chills or hypotension Two positive blood cultures for a common skin contaminant (from two separate blood samples, usually within 48 hours). Examples of skin contaminants are coagulase-negative staphylococci, Micrococcus sp., Propionibacterium acnes, Bacillus sp. and Corynebacterium sp.If common skin contaminants are found in the blood culture, a confirmation (i.e., same microorganism with same resistance patterns, within 48 hours) is needed from a second blood sample to be sure that the finding is not based on a contaminated sample.
The table below lists the most important and common pathogens identified in blood by the ECDC. These may, however, be different for your hospital or local context.
Recognized pathogens Common skin contaminants Staphylococcus aureus Coagulase negative staphylococci (CoNS) Enterococcus spp. Corynebacterium sp. Escherichia coli Bacillus sp. Klebsiella spp. Propionibacterium acnes Pseudomonas spp. Micrococcus sp. Acinetobacter spp. Candida spp. If microbiology laboratory support is unavailable or the blood culture was not done or is negative, the definition of unidentified severe infection (formerly “clinical sepsis”) can be used, according to the following criteria:
- Patient has at least one of the following clinical signs or symptoms with no other recognized cause: fever, hypotension or oliguria and, for infants ≤1 year, fever, hypothermia, apnea or bradycardia.
- A blood culture is not done or is negative.
- There is no apparent infection at another site.
- The physician institutes treatment for sepsis.
The following definitions are infections associated with the insertion site but do not cause BSI. Click or tap on each tab to learn more.
Thrombophlebitis
Thrombophlebitis is a process of local vein inflammation and thrombus formation. There must be damage to vascular integrity (mechanical or irritating drugs), though it is not an infection. It is associated with peripheral venous catheter only. Local signs include redness, swelling, tenderness, pain, warmth and palpable cord/vein.3
Catheter exit-site infections
Catheter exit-site infections (also called local infections) are caused by skin organisms that migrate into the skin tract of the catheter. They are associated with bacterial skin colonization at the insertion site. CoNS are the most common types of skin contaminants, but S. aureus are the most dangerous.
These types of infections are associated with both peripheral (intravenous lines placed in the hand, arm or feet) and central (lines placed in central veins usually in the neck or upper chest) venous catheters. There are two case definitions for catheter exit-site infections. These definitions have set criteria that must be met in order to be deemed a catheter exit-site infection. Click or tap on the tabs below to learn about each definition.
Definition 1
Peripheral venous catheter (PVC)-related infection (CRI1-PVC) = local PVC-related infection (no positive blood culture).
CRI = catheter related infectionThe following criteria must be met:Positive quantitative PVC culture ≥10^3 colony-forming unit (CFU)/mL semi-quantitative PVC culture >15 CFU pus/inflammation at the insertion site or tunnelDefinition 2
Cardiovascular (arterial or vascular) infection (CVS-VASC). At least one of the following criteria must be met:- patient has organisms cultured from arteries or veins removed during a surgical operation and blood culture not done or no organisms cultured from blood;
- patient has evidence of arterial or venous infection seen during a surgical operation or histopathologic examination;
- patient has at least one of the following signs or symptoms with no other recognized cause: fever (>38°C), pain, erythema or heat at involved vascular site; more than 15 colonies cultured from intravascular cannula tip using semi-quantitative culture method; blood culture not done or no organisms cultured from blood;
- patient has purulent drainage at involved vascular site; blood culture not done, or no organisms cultured from blood. This criterion is purely clinical and allows identification without microbiology.
The figure below helps to describe the process or mechanisms by which thrombophlebitis and exit-site infections occur in PVCs.
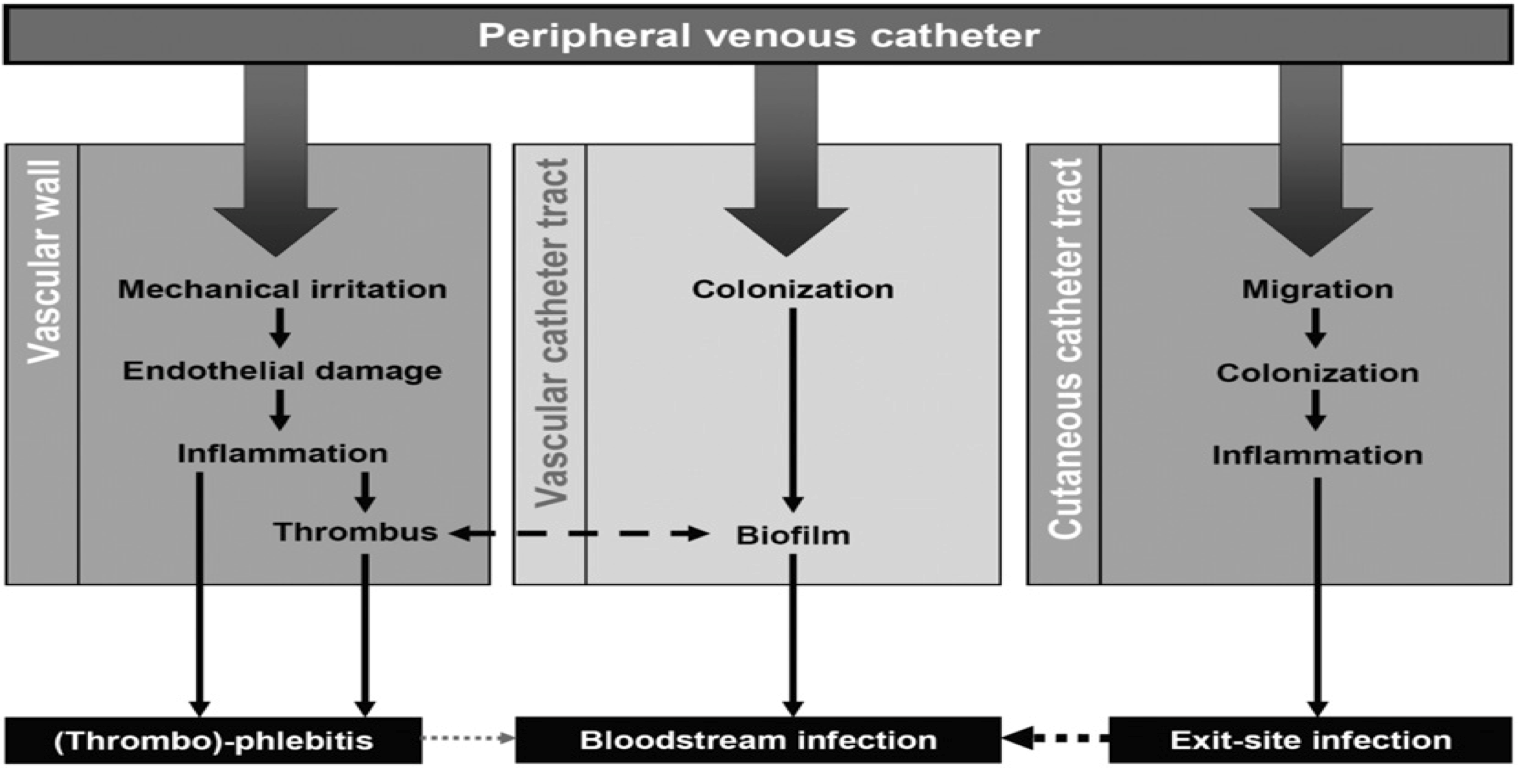
Source: Zingg W, Pittet D. 2009.
-
Definitions of Catheter-Associated vs Catheter-Related BSI (5 min)
Let us look at the difference between catheter-associated BSI and catheter-related BSI (CRBSI).
A catheter-associated BSI is the link between the infection and the catheter based on time; the catheter is in place at the same time or 48 hours before (according to the ECDC definition) or the day before the diagnosis of BSI (according to the CDC definition). On the other hand, for CRBSI, the link between infection and catheter is clearly established microbiologically. This means the presence of infection originates from the catheter. The definitions for CRBSI presented below are only applied by ECDC.
Peripheral line tips are rarely sent for culture; thus, for peripheral lines you are more likely to find catheter-associated infections rather than catheter-related infections.
Click or tap on each tab to read the definitions.
Catheter-associated infections
Definitions for BSI (i.e., a positive blood culture result) are met as described above.ECDC: a peripheral or central catheter is in place in the 48 hours preceding the onset of infection.
CDC: an eligible BSI organism is identified, and a central line is present on the day of laboratory confirmed BSI diagnosis or the day before = central line associated BSI.3
CVC-related infections (ECDC)
CRI1-CVCLocal (at the insertion site) CVC-related infection (no positive blood culture)
Positive quantitative CVC culture ≥10^3 CFU/mL semi-quantitative CVC culture >15 CFU pus/inflammation at the insertion site or tunnelCRI2-CVCGeneral CVC-related infection (no positive blood culture)
Positive quantitative CVC culture ≥10^3 CFU/mL semi-quantitative CVC culture >15 CFU clinical signs improve within 48 hours after catheter removalCRI3-CVCMicrobiologically confirmed CVC-related bloodstream infection
BSI occurring 48 hours before after catheter removal (if any) positive culture with the same microorganism of either:- quantitative PVC culture ≥10^3 CFU/mL or semi-quantitative PVC culture >15 CFU
- quantitative blood culture ratio of CVC blood sample/peripheral blood sample >5
- differential delay of positivity of blood cultures: CVC blood sample culture positive two hours or more before peripheral blood culture (blood samples drawn at the same time)
- positive culture with the same microorganism from pus from insertion site
PVC-related infections
With these types of infections there is a formal relation between infection and catheter. The definition is:
CRI1-PVC(also reported above as Catheter exit-site infection local PVC-related infection)
Local PVC-related infection (no positive blood culture)
Positive quantitative PVC culture ≥10^3 CFU/mL semi-quantitative PVC culture >15 CFU pus/inflammation at the insertion site or tunnelCRI2-PVCGeneral PVC-related infection (no positive blood culture)
Positive quantitative PVC culture ≥10^3 CFU/mL or semi-quantitative PVC culture >15 CFU clinical signs improve within 48 hours after catheter removalCRI3-PVCMicrobiologically confirmed PVC-related infection (no positive blood culture)
Bloodstream infection occurring 48 hours before after catheter removal (if any) positive culture with the same microorganism of either:- quantitative PVC culture ≥103 CFU/mL semi-quantitative PVC culture >15 CFU;
- positive culture with the same microorganism from pus from insertion site
-
Epidemiology of BSI (5 min)
BSIs—and health care-associated infections (HAIs) in general—place a significant burden on the health care system. HAIs result in longer hospital stays, which reduces the number of beds available for new patients, require costlier medications to treat and can lead to premature death.
In the USA and Europe, BSIs usually count for about 9-11% of all HAIs hospital-wide; in low- and middle-income countries (LMICs) this proportion is usually higher (19%).4, 5
Although BSIs do not occur as often as many other HAIs, they have a higher impact on patient outcomes; it is the second most impactful HAI (after pneumonia) in terms of disability life years lost due to its consequences. Furthermore, BSIs inflict a very high burden on the health care system. A large study conducted in Europe found that patients with BSIs stay in the hospital an additional 6 to 11.5 days, and attributable costs from BSIs range from US$ 8,000 to US$ 56,000.6, 7, 8
Crude mortality rate from BSIs has been shown to be 10-40%, with an attributable mortality of 7-20%.6 If the infection is resistant to antibiotics, this burden increases, sometimes significantly.
Burden of BSI in LMICs
In contrast to infections associated with PVCs, there exists more evidence for central line-associated BSI (CLABSI). Pooling data published between 1995 and 2010 WHO estimated that the incidence of CLABSI in LMICs was four times higher than in high-income countries (12.2/1,000 catheter days as compared to 3.5/1,000 catheter days).4
In a large study conducted by the International Nosocomial Infection Control Consortium (INICC) in 45 LMICs, rates of CLABSI were significantly higher in the intensive care units (ICUs) of the INICC hospitals compared to ICUs in the USA.9
ICU Type CLABSI Rate INICC 2012-2017 Pooled Mean (95% CI) US NHSN 2013 Pooled Mean (95% CI) Medical 4.47 (4.1-4.8) 1.1 (1.0-1.2) Paediatric 7.19 (6.7-7.7) 1.2 (1.1-1.3) Surgical 5.23 (4.7-5.6) 0.9 (0.8-1.0) Source: Adapted from Rosenthal VD 2019.
In the same study, the authors found that the most common pathogens isolated from CLABSI were highly resistant to antibiotics, with resistance proportions often significantly higher than those reported in the USA (for instance carbapenem-resistance among P. aeruginosa and K. pneumoniae).
Pathogen CLABSI No. of Pathogenic Isolated Tested at INICC ICUs, Pooled Resistance Percentage at INICC ICUs, % Resistance Percentage at CDC NSHN ICUs, % Staphylococcus aureus OXA 51 64.7 50.7 Enterococcus faecalis VAN 27 18.5 9.8 Pseudomonas aeruginosa FQs 110 20.0 30.2 PIP or TZP 91 33.0 18.4 AMK 112 21.4% 10.0% IPM or MEM 92 43.48% 26.1% FEP 60 41.67% 26.1% Klebsiella pneumonia CRO or CAZ 191 67.54% 28.8% IPM, MEM or ETP 205 36.10% 12.8% Acinetobacter baumanii IPM or MEM 128 73.44% 62.6% Escherichia Coli CRO or CAZ 85 52.94% 19.0% IPM, MEM or ETP 81 8.64% 1.9% FQs 81 49.38 49.3 Source: Adapted from Rosenthal VD 2019.
Among device-associated infections, CLABSI are those carrying the highest in LMICs (41.6% and 32% in adult/paediatric ICUs and in neonatal intensive care units [NICUs], respectively). In a study conducted at a teaching hospital in Brazil, the impact of hospital acquired methicillin-resistant S. aureus (MRSA) BSI was 32.1 days excess length of stay, 45.2% excess mortality and increased hospital expenditures (up to US $123,065 for cases vs US $40,247 for controls). Additionally, the antimicrobial therapy cost was 6.7 times higher for cases than controls.10
In one study, central venous catheter infections were shown to increase ICU admissions length of stay by 10 days on average in adults and pediatrics and 19 days for neonates.11
-
Your Hospital or Setting (5 min)
It’s important for you know what the burden of BSIs is in your hospital. If the information is available, find out:
- How many patients get peripheral or central venous catheters?
- What is the most common adverse event due to the use of peripheral or central venous catheters?
- How frequent are BSIs due to the use of peripheral or central venous catheters?
-
Infections Associated With Peripheral Lines (5 min)
It is estimated that 30–80% of patients receive a peripheral line during their hospital stay. PVCs are likely the most used device in a health care setting due to their wide use. Click or tap on the circles in the graphic below to learn about how frequently BSIs occur in peripheral catheters compared to other adverse events.3, 12, 13
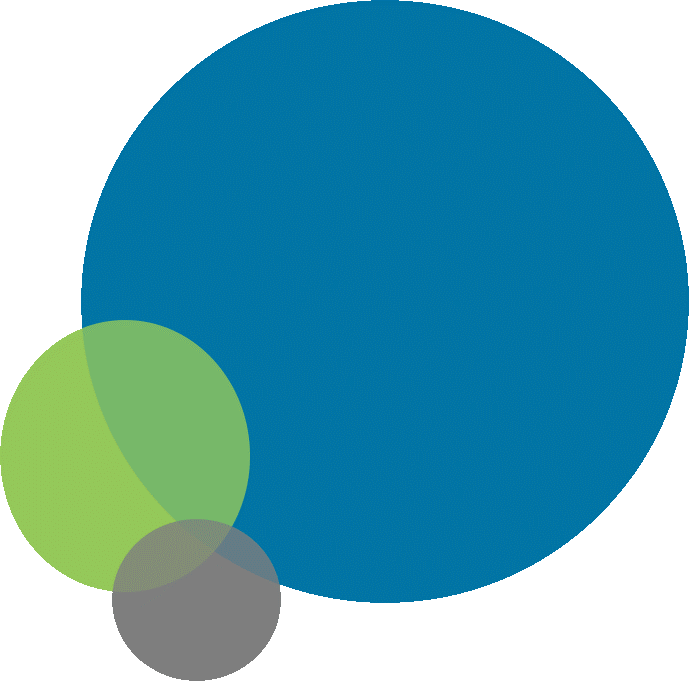
Blue circle
The most common (20–80%) adverse event from using peripheral lines is phlebitis, or thrombophlebitis if associated with an intravascular thrombus. Phlebitis is not an infection, so it does not need antimicrobials.
Green circle
Exit-site infections are less common and occur about 2–7% of the time.
Gray circle
BSI in PVCs are rare and occur less than 1% of the time, but they are also probably underestimated because they are not detected. The incidence density of BSIs due to the use of PVCs is estimated at 0.2 to 0.7 episodes per 1,000 device days—lower than rates from central lines and higher than rates from fully implantable medical devices such as ports. Incidence density is used to report CRBSI because the catheter dwell time is reported to be the main risk for infection. The longer the catheter in place, the higher the risk. If there is no catheter, there cannot be a CRBSI!
Unfortunately, there is not much scientific literature about infections associated with PVCs. Here are data from a few published studies:
- In Egypt, researchers followed 261 lines on 83 newborn patients in a NICU. They found 45 episodes of sepsis were diagnosed from clinical and/or blood cultures.14
- In Trinidad and Tobago, researchers observed about 8 BSIs per 100 patient admissions, of which 80% had IV lines.15
- In Uganda, PVC tips and blood cultures were collected from 391 paediatric patients 81 (21%) of the tips were colonized with organisms, 23 (6%) had BSIs with the same organisms as in the tips and 77 (17%) had phlebitis.16
- In Brazil, a study among 122 patients with PVCs found that 38 (31%) developed phlebitis.17
- In Tunisia, 312 patients were followed at a university hospital. PVC was determined as one of three independent risk factors of HAIs (OR 3.5, 95% CI 1.3–9.4).18
- In Cambodia, an outbreak of Burkholderia spp was reported due to Ringer lactate for catheter flushing.19
The graphic shows the highest to lowest rates of BSI due to catheter-related use.20
-
Risk Factors (5 min)
Now we will look at risk factors for catheter-associated BSI. Pathogens can be spread from patient to patient via contamination of health care worker (HCW) hands, which may be the cause of catheter contamination in up to 60% of cases. Patients can be infected by their own microorganisms if the HCW placing the catheter does not observe the appropriate antiseptic technique.
Contaminated fluids and drugs are another source of infection of the bloodstream and catheter; this rarely occurs in high-income countries, but still may be an issue in LMICs due to the use of glass bottles, open systems, and unsafe preparation of admixtures (e.g., for parenteral nutrition) in the in dirty environments in the wards.
In LMICs, insertion of the catheter without sterile equipment and aseptic technique is common malpractice. Furthermore, gaps in catheter management are very common, including, for example, absence of or a dirty dressing and contamination of the hubs during drug injection through the catheter.
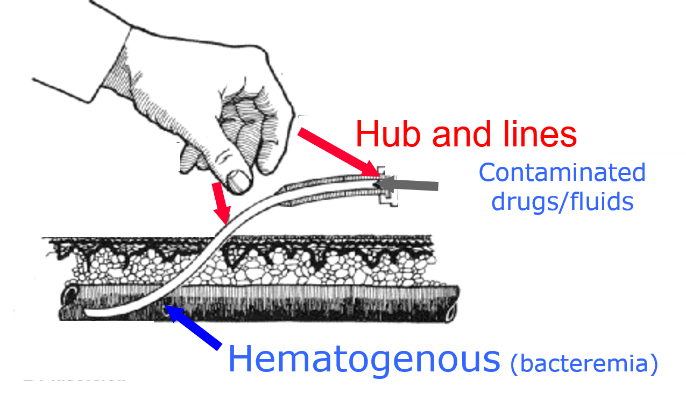
Examples of practices that increase the risk of CRBSI in health care settings include:
- cotton balls already impregnated with contaminated antiseptic;
- central line in place with no dressing;
- open semi-rigid intravenous container with administration set and three-way stopcock for intravenous preparation;
- open vials covered with contaminated tape;
- peripheral line in place, with no sterile dressing;
- one-use vials used multiple times; and
- semi-rigid plastic container used for intravenous preparation.
Risk factors linked to the patient include:
- conditions that determine immunosuppression (both diseases and therapies)
- severe and/or multiple underlying diseases
- under- or malnutrition
- age (elderly patients, neonates)
- low birth weight
- multiple invasive procedures.
Unnecessary placement of catheters represents another factor leading to the risk of BSI. Try to avoid using catheters whenever a suitable alternative is available. The table below shows possible alternatives to using a catheter.
Indication Possible Alternative Multiple blood draw Venipunctures Fluid administration Nasogastric tube Continuous drug Subcutaneous route Multiple drugs Oral/rectal route Emergency drugs Oral/inhalation Monitoring SpO2 Blood pressure, SaO2 Cuff Note that if one or more indications for catheter use are present and no safe alternative is possible, catheter use is indicated.
The right kind of catheter depends on how long you expect the patient will need to have the catheter. If the catheter is needed for six days or less, use a peripheral catheter if possible. If the patient requires a catheter for more than six days, use a CVC or a peripherally inserted central catheter (PICC) as extended peripheral catheter use can cause thrombophlebitis.
A peripherally inserted central catheter, less commonly called a percutaneous indwelling central catheter, is a form of intravenous access that can be used for a prolonged period of time or for administration of substances that should not be given peripherally.Place peripheral lines in the wrist, middle arm, external jugular or foot. Place PICC lines in the middle arm, humeral or leg. CVC lines can be placed in the femoral, jugular or subclavian veins. If the line will be needed for longer than five days, change CVC lines placed in subclavian veins after three to five days. The subclavian vein is the safest access point because it is less likely to be contaminated than the jugular vein—e.g., from drool or saliva.
In emergency situations, access must be fast and safe. It is crucial to prevent or minimize other complications, including aerial puncture and pneumothorax. In case of shock, an easy, quick and reliable access is important.
-
Knowledge Check (10 min)
-
Summary (5 min)
This module covered case definitions of BSI, including exit-site infections, catheter-associated infections, central catheter-related infections and peripheral catheter-related infections. You also learned that BSI, while a small proportion of all HAI, place a significant burden on the health care system due to longer hospital stays, increased costs, and premature death. Finally, you learned about the factors that place patients at a higher risk for acquiring BSI. These include contaminated hands, not using dressings to cover exit sites, and reusing single-use items. Whenever possible, reduce the chance that a patient will contract a BSI by observing proper IPC practices (such as performing hand hygiene) and using suitable alternative to catheters when possible.
-
References
- European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals – protocol version 5.3. Stockholm: ECDC; 2016.
- Centers for Disease Control and Prevention/National Healthcare Safety Network. Surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. 2019.
- Zingg W, Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34 Suppl 4S: S38-42. doi:10.1016/S0924-8579(09)70565-5.
- Report on the burden of endemic health care-associated infection worldwide. Geneva: World Health Organization; 2011.
- Suetens C, Latour K, Karki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46).
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56-66. doi:10.1016/S1473-3099(18)30605-4.
- Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducombe T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150;
- Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23:17-00454.
- Rosenthal VD, Bat-Erdene I, Gupta D, Belkebir S, Rajhans P, Zand F, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: Device-associated module. Am J Infect Control. 2019 Oct 29. pii: S0196-6553(19)30795-3. doi: 10.1016/j.ajic.2019.08.023.
- Borges Primo MG, Guilarde AO, Martelli CM, Batista LJ, Turchi MD. Healthcare-associated Staphylococcus aureus bloodstream infection: length of stay, attributable mortality, and additional direct costs. Braz J Infect Dis. 2012;16:503–509.
- Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004--2009. Am J Infect Control 2012;40:396-407. doi:10.1016/j.ajic.2011.05.020.
- Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med 1998;158(2):151-6. doi:10.1001/archinte.158.2.151.
- Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev 2015;(8):CD007798.
- Bakr AF. Intravenous lines-related sepsis in newborn babies admitted to NICU in a developing country. J Trop Pediatr. 2003; 49(5):295-297. doi:10.1093/tropej/49.5.295.
- Orett FA, Brooks PJ, Richardson EG. Nosocomial infections in a rural regional hospital in a developing country: infection rates by site, service, cost, and infection control practices. Infect Control Hosp Epidemiol. 1998; 19(2):136-40. doi:10.1086/647781.
- Nahirya P, Byarugaba J, Kiguli S, Kaddu-Mulindwa D. Intravascular catheter related infections in children admitted on the paediatric wards of Mulago Hospital, Uganda. Afr Health Sci. 2008;8(4):206–216:PMC2887016.
- Enes SM, Opitz SP, Faro AR, Pedreira Mde L. Phlebitis associated with peripheral intravenous catheters in adults admitted to hospital in the Western Brazilian Amazon. Rev Esc Enferm USP. 2016;50(2):263-71. doi:10.1590/S0080-623420160000200012.
- Mahjoub M, Bouafia N, Bannour W, Masmoudi T, Bouriga R, Hellali R, Ben Cheikh A, Ezzi O, Ben Abdeljellil A, Mansour N. Healthcare-associated infections in a Tunisian university hospital: from analysis to action. Pan Afr Med J 2015;20:197. doi:10.11604/pamj.2015.20.197.4062.
- De Smet B, Veng C, Kruy L, Kham C, van Griensven J, Peeters C, Ieng S, Phe T, Vlieghe E, Vandamme P, Jacobs J. Outbreak of Burkholderia cepacia bloodstream infections traced to the use of Ringer lactate solution as multiple-dose vial for catheter flushing, Phnom Penh, Cambodia. Clin Microbiol Infect. 2013;19(9):832-7. doi:10.1111/1469-0691.12047.
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81(9):1159-71. doi:10.4065/81.9.1159.

