Decontamination & Sterilization Part 1
The decontamination of instruments and medical devices plays a very important role in the prevention of health care-associated infections (HAIs). Indeed, improper decontamination of surgical instruments, endoscopic devices, respiratory care devices and reusable haemodialysis devices still occurs in many settings, leading to HAIs. In addition, in many low-resource settings, reuse of single-use medical devices is common practice. In this module, you will learn about risk assessment as it relates to reusable medical devices, the dangers of reusing single-use devices, and the requirements for a decontamination unit.
Learning Objectives
By the end of this module, you will be able to:
- describe decontamination and Spaulding classification;
- explain the dangers of reprocessing single-use medical devices;
- describe the layout and flow of the decontamination unit; and
- list the steps for proper receipt, storage and transportation of sterile medical devices.
Learning Activities
-
The Sterile Services Department (SSD) (5 min)
A midwife at your medical centre has just finished safely delivering a newborn baby girl. You have been asked to take care of all reusable medical devices used during labour (forceps, scalpel, scissors, needle driver, vacuum) for safe reprocessing. On a piece of paper or in the box below, write 4-5 sentences describing the decontamination process for these devices. Some questions to ask yourself might be: How do I safely transport them from the birthing unit to the SSD? In the SSD, where would you take the dirty devices first? What are the steps for decontamination? Can all items be reprocessed for re-use? What equipment does your facility have to process these devices?
As you learn about decontamination and sterilization, compare what you learn to how you described reprocessing the devices in the childbirth kit.
-
What Is Decontamination? (5 min)
Before going further, we should define some key terms that will be used throughout this module.
Decontamination is the process of removing soil and pathogenic microorganisms from objects, such as medical devices, so they are safe to handle, whether that involves further processing (sterilization), use or disposal. There are three parts to decontamination: cleaning, disinfection and sterilization.
Note that in the United States, the term ‘decontamination’ generally refers to only disinfection and/or sterilization; the cleaning step is excluded. In the United Kingdom and Europe, ‘decontamination’ refers to the entire process, including cleaning, disinfection and/or sterilization; this is the definition we will be using.
Let us define what a medical device is.
To determine the level of decontamination required for a particular medical device, it is important to understand the differences between cleaning, disinfection and sterilization. Click or tap on each tab to read the definition.
Cleaning
The first step in the decontamination process is cleaning, which is required to remove contamination by foreign material, such as dust or soil. Cleaning also removes organic material, such as blood, secretions, excretions and microorganisms, to prepare a medical device for disinfection or sterilization. You cannot perform proper disinfection or sterilization of any item without first doing a thorough cleaning.
Disinfection
Disinfection is the process of reducing the number of viable microorganisms to a less harmful level. This process may not inactivate bacterial spores, prions and some viruses.
Sterilization
Sterilization is a validated process used to render an object free from viable microorganisms, including viruses and bacterial spores, but not prions.
This module is based on the WHO Manual on Decontamination and Reprocessing of Medical Devices for Health-care Facilities and the CDC Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Both documents are available in the Resources section.
-
Assessing Risk (5 min)
To assess the risk of reusing a medical device, consider the following four factors:
- The type of item or device: critical, semi-critical or non-critical.
- Type of microorganism: bacteria, spores, viruses or prions.
- Presence of microorganisms in number (bioload) and availability to cause infection.
- Patient susceptibility (whether the type of procedure is invasive or non-invasive).
The Spaulding classification system was developed in the 1950s to help people decide which method of decontamination was appropriate for different types of reusable devices, based on the potential risk of infection posed to a patient. Spaulding divided medical devices into three risk categories: high risk (critical), intermediate risk (semi-critical), and low risk (non-critical). Click or tap on each tab to learn more about each type of risk.
High risk (critical)
Definition: Medical devices that are involved with a break in the skin or mucous membrane or enter a sterile body cavity.
Method of decontamination: Sterilization, usually heat (if heat-stable), or chemical (if heat-sensitive). Heat-stable items may be treated using low-temperature steam and formaldehyde, ethylene oxide, or radiation.
Examples of common items/equipment: Surgical instruments, implants, prostheses and devices, urinary catheters, cardiac catheters, needles and syringes, dressings, sutures, delivery sets, dental instruments, rigid bronchoscopes, cystoscopes.
Intermediate risk (semi-critical)
Definition: Medical devices in contact with mucous membranes or non-intact skin.
Method of decontamination: High-level disinfection by heat or chemical (under controlled conditions with minimum toxicity to humans).
Examples of common items/equipment: Respiratory therapy and anaesthetic equipment, flexible endoscopes, vaginal specula, reusable bedpans and urinals, urine bottles, patient wash bowls.
Low risk (non-critical)
Definition: Items in contact with intact skin.
Method of decontamination: Low-level disinfection (i.e., cleaning with detergent, with or without disinfectant).
Examples of common items/equipment: Blood pressure cuffs, stethoscopes, electrocardiogram leads, occupational therapy tables and environmental surfaces.
-
Single-Use Reprocessing (15 min)
Use your best guess to answer these questions.
High-Risk Classification
Even within the ‘high risk’ category, some items are very high risk. These items are very difficult to clean and are heat-sensitive. If reused, they can cause serious infections in patients, often with severe consequences. Click or tap on each tab to read about classes of high risk.
Class 1: Very high risk
Reuse can cause serious infections, such as endocarditis, meningitis, endophthalmitis and transmission of blood-borne viral infections. Very high-risk devices, which include intravascular, intraventricular, and intraoptic devices and needles and syringes, are difficult to clean and heat-labile. These devices must never be reprocessed and reused!
Class 2: High risk
These types of devices are usually cleanable and heat-tolerant. Sterilization (such as for surgical instruments) is necessary with these devices, if deemed safe to reprocess.
Dangers of Reusing Single-Use Devices
The reuse of devices intended for single use is a safety issue: reuse can cause transmission of infection. Some devices cannot be appropriately or safely decontaminated, as some types of surfaces—e.g., those with acute angles, coils, long or narrow lumens—can be difficult to access, and some types of equipment have special surface coatings that may hinder or compromise the sterilization process. Ultimately, it is not possible to completely validate removal of all microorganisms, which is necessary to achieve effective decontamination. Any person who reprocesses or reuses a device intended by the manufacturer for single use bears full responsibility for its safety and effectiveness.
If Single-Use Devices Must Be Reused
Reuse of single-use medical devices is a common, inappropriate practice worldwide. However, before reusing a device, conduct a risk assessment first. In situations, where reuse of single-use devices does occur, it is important to weigh the costs and benefits of this practice. Sometimes reuse is necessary because there is no alternative or additional device for use. These should be exceptional situations; every possible effort should be made to advocate for and achieve timely and sufficient procurement of single-use devices necessary for use in the facility. If you must reuse a device, ask “How can this be done in the safest way to reduce any potential risk?’ The points listed here are part of an overall strategy aimed at reducing the potential for a negative outcome when extraordinary reasons demand the re-use of single-use devices:
- no alternative is available;
- the device is solid (i.e., without a hollow lumen);
- the method of reprocessing is compatible with the device;
- written reprocessing procedures are available for each type of single-use device;
- validation of the effectiveness of reprocessing procedures is undertaken to ensure both sterility and functionality of the device;
- records are kept in detailed log books (located within the SSD) containing patient and device information in case of performing a look-back exercise;
- the device is inspected each time for fractures, stress and loss of integrity, and discarded if not fit for use; and
- a note is made in the patient’s file that a reprocessed single-use device was used.
-
Knowledge Check (5 min)
-
Decontamination Life-Cycle (5 min)
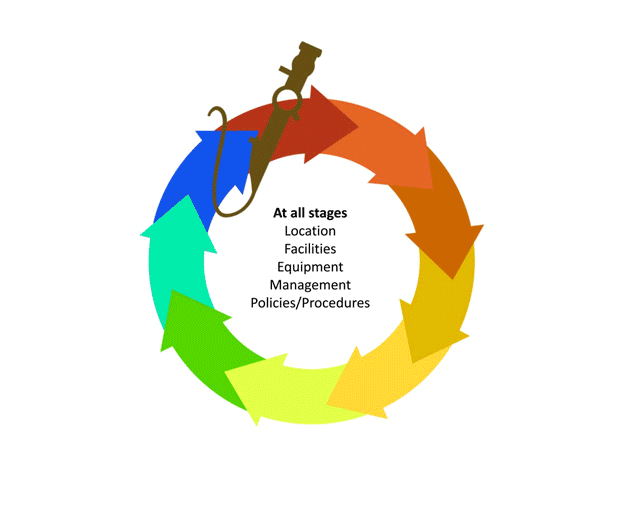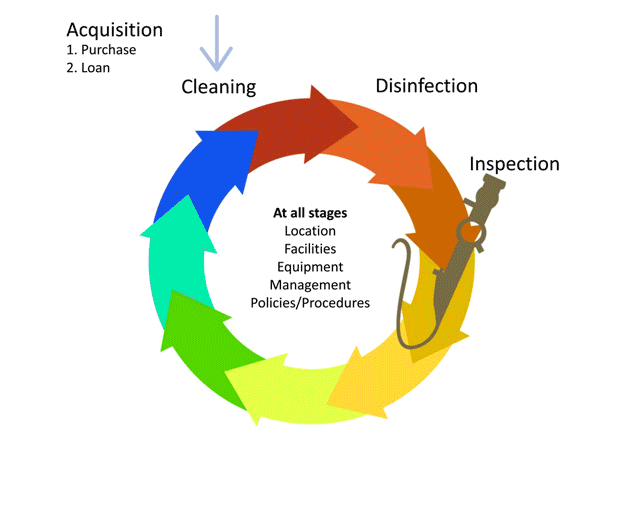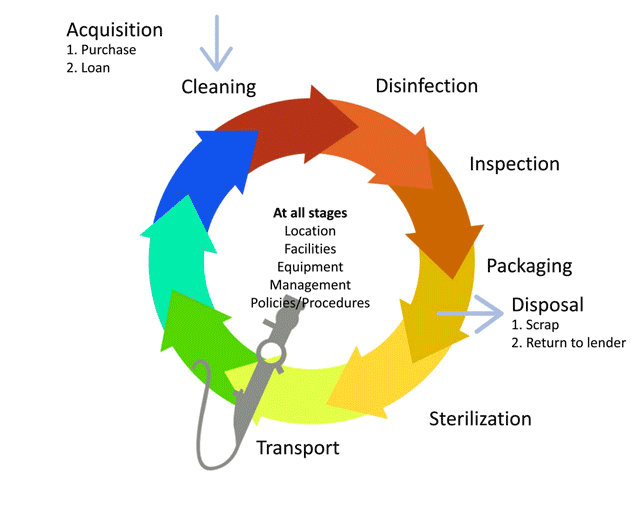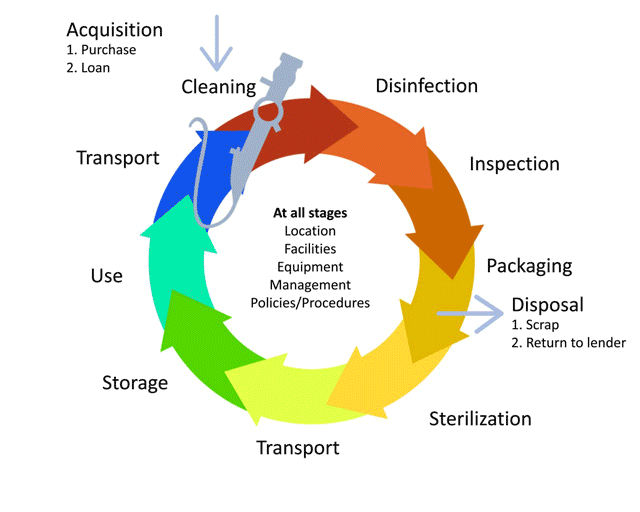
Before we learn about the layout, flow and workforce within an SSD, let’s look at the decontamination life-cycle.
The life-cycle of decontamination illustrates the relevant features of decontamination, with each step being as important as the next. This cycle represents each stage of the decontamination process, beginning from point of use or acquisition to decontamination followed by storage. This cycle is continuous and demands a minimum standard at every step to ensure safe reuse and handling of medical devices. Note that cleaning is important, as it is the basis for proper decontamination.
As we move through the decontamination modules, we will examine each step in more detail and follow the life of a medical device.
Watch this short animation on the decontamination life-cycle.
-
Sterile Services Department Layout (5 min)
WHO recommends implementing IPC interventions and practices using multimodal strategies (see the module on Core Components and Multimodal Strategies). One element of these strategies is system change (the ‘build it’ component); for decontamination, this means ensuring that the infrastructures, layout and equipment of your SSD are adequate.
Click or tap on each number to learn more about the layout of the SSD. Note that the SSD must have restricted access.
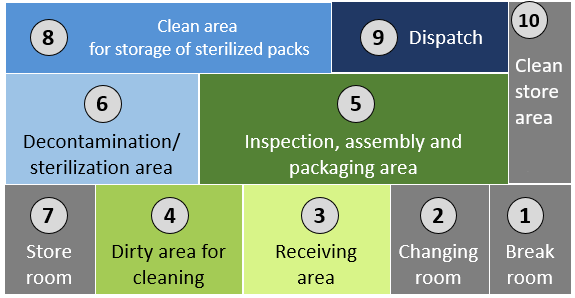
1
Break room for SSD staff.
2
Changing room to put on PPE before entering specific work areas.
3
Receiving area of used instruments and medical devices that are to be cleaned.
4
Dirty area for cleaning used instruments and medical devices.
5
Inspection, assembly and packaging area for instruments and medical devices.
6
Decontamination/sterilization area where instruments are loaded for sterilization and unloaded after the sterilization cycle is completed.
7
Clean Store room for material used in SSD.
8
Clean storage area where sterilized items are stored.
9
Dispatch area where items are dispatched to the wards/units and clinical areas.
10
Clean area for storage of linen, PPE and other materials used in the SSD.
The concept of flow of medical devices from dirty to clean must be maintained, even if only one room is available. Clear delineation between clean and dirty is required to avoid recontamination after decontamination of reusable medical devices. Changes can be made to improve overall flow, but when building or renovating a new SSD, it is important to reference and use national/international recommendations.
-
Environment (10 min)
The environment of the inspection, assembly and packaging area should be distinctly separate from areas where clean, disinfected and sterile devices are handled or stored, and it should have restricted access. These areas should be set up in a way to ensure a one-way work flow of staff and medical devices. There should be adequate space for the packing process and storage of necessary equipment and supplies. All spaces used for reprocessing of medical devices must have hand hygiene facilities at both entrance and exit points.
Environmental Controls
Environmental controls help maintain sterility in the SSD.
Click or tap on each icon to learn about the various environmental controls in an SSD.
Air
Air supplied to the SSD should be of medical quality (i.e., free of bacteria, chemicals and large particles of dust or dirt).
Water
The quality of water is integral to the cleaning process and to produce steam for sterilizers. Water should be of high quality in the sterile services department. Ideally, water used in the SSD should be soft, which means that the mineral and salt content is low and will not affect devices or processing equipment. If the water supplying this unit is not soft, consider water-softening methods, such as filtration, distilling, ion exchange and reverse osmosis.
Ventilation
The dirty area should be under negative-pressure ventilation in relation to the clean areas, which should be under positive-pressure ventilation. It is essential that working conditions are comfortable for staff, as they will spend the whole day in a confined space. It is best not to use a fan with an open window to create airflow, as this will lead to contamination or recontamination of medical devices. Air should flow from clean to dirty areas to minimize contamination of medical devices.
Humidity
The recommended relative humidity level is 40–50%.
Ambient temperature
A therma-hygrometer facilitates more accurate monitoring of environmental controls, as it can measure both temperature and humidity. You can set up a therma-hygrometer with a log to record its readings. In some places, these devices are automated and can alert staff when environmental control requirements are not met. Alternate solutions are available, such as separate thermometers and hygrometers.
The following temperatures are recommended:
- Decontamination areas: 18–20 °C
- Clean areas: 18–23 °C
- Sterile storage areas: 15–25 °C
-
Flow (5 min)
Now that you have learnt about the SDD layout, we will look at some best practices for workflow. The concept of flow of medical devices from dirty to clean must be maintained. This objective cannot be achieved in a one-room setup! Ten rules govern the SSD’s location.
- It should be designed to be physically separate from all other work areas and should not interfere with routine clinical practice.
- It should not be an integral part of any other service or treatment area, such as an operating theatre.
- It should not be used as a passageway.
- It should be purpose-specific and built for reprocessing devices, with clearly demarcated areas.
- It should be designed to allow the segregation of ‘dirty’ and ‘clean’ activities.
- It should be designed to facilitate one-way flow from the dirty area to the clean area.
- It should have a dedicated staff area close by for changing into workwear; the staff area should include a shower, toilet facilities and lockers.
- Access to dirty and clean areas – such as the inspection, assembly and packaging room – should be through separate, dedicated gowning rooms provided with hand hygiene facilities.
- The dirty, inspection, assembly and packaging, sterilizing and sterilizer unloading areas should be free of windows that can be opened, ledges and difficult-to-clean areas.
- The dirty, clean, inspection, assembly, and packaging and sterilizing areas should be designed to minimize ambient sound levels within the rooms. This requires particular attention to installation of equipment, building finishes and maintenance of machines.
To understand how these rules apply to the overall functioning of an SSD unit, click or tap on the numbers in the graphic below, starting with 1.
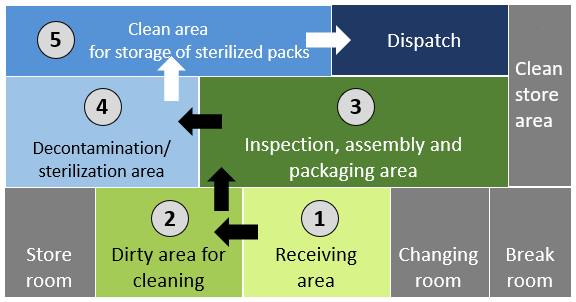
1
Instruments and medical devices are delivered to the receiving area.
2
They are brought into the dirty area and cleaned (either manually or mechanically).
3
Once cleaned, they are passed through the inspection, assembly and packaging area. Instruments and medical devices can only be returned from this area to the receiving area if they have not been properly cleaned.
4
After packaging, they are put into the sterilizer. Sterilized items are taken out.
5
Stored in a clean area to await dispatch to clinical areas.
-
Staff Training (5 min)
In addition to having proper layout and workflow within the SSD, staff must be trained at the appropriate levels of activity, including using PPE and how to handle chemicals safely. This is part of the ‘teach it’ component of the multimodal strategy.
Make sure the training covers all aspects of the decontamination cycle and provides technical training on all reprocessing equipment. Staff must also be trained to recognize problems and interpret validation tests (i.e., recognize failed results). When working in an SSD, it is imperative that staff are trained on occupational hazards and how to manage them. Moreover, staff working in this area require hepatitis B immunization because of the constant exposure to blood-borne pathogens. Taking such preventive measures will help to reduce and eliminate occupational threats.
-
Environmental Cleaning (5 min)
The last item we will cover for setting up the SSD unit is environmental cleaning. It is important to have an environmental cleaning policy for the SSD. Make sure that the policy is clearly defined by the health care facility, with clear SOPs that cover cleaning practices, who is responsible for cleaning, and cleaning frequency.
To verify environmental cleaning, use this checklist with an associated validation method.
- Cleaning should be performed daily, moving from clean to dirty areas, and keeping the environment clean, dry and dust-free.
- Separate cleaning equipment should be used for clean and dirty areas.
- The use of disinfectants for routine cleaning is strongly discouraged.
- Disinfectants are recommended only when dealing with spills.
- All leaks and spills must be cleaned up immediately.
For further information, see the Standard Precautions Environmental Cleaning module.
-
Receipt, Storage and Transportation of Medical Devices (5 min)
We will move now to the first main stage of the decontamination cycle—the receipt, storage and transport of medical devices to the SSD. Before sending medical devices to the SSD for cleaning, be sure to wear the appropriate PPE to protect yourself. Remove any linen and disposable items and dispose of sharps, such as knife blades and needles, in puncture-proof sharps containers or bins.
Do not use saline or hypochlorite solution as a soaking solution as this will damage some medical devices. Soaking devices in 0.5% chlorine solution or any other disinfectant before cleaning is not recommended (strongly discouraged) because:
- it may damage or corrode the devices;
- the disinfectant may be inactivated by blood and body fluids, which could then become a source of microbial contamination and biofilm formation;
- transport of contaminated items soaked in chemical disinfectant to the decontamination area may pose a risk to health care workers and result in inappropriate handling and accidental damage; and
- soaking in disinfectant could contribute to the microorganisms developing resistance to disinfectants.
Soiled medical devices should be opened and kept moist. Spray them with an enzymatic spray (using PPE) and cover with a towel moistened with either water (not saline) or a foam, spray or gel specifically intended for this purpose.
Transport of Medical Devices
Contaminated items should be kept in dedicated, fully enclosed, leak- and puncture-proof containers prior to transport to the decontamination area in a transportation trolley. Do not transport in containers with water, as water is a splash hazard.

-
Knowledge Check (5 min)
-
Summary (5 min)
This module covered the different components of the decontamination process, which are cleaning, disinfection and sterilization. You learnt how to use the Spaulding classification to assess the risk of infection when decontaminating medical devices. We also discussed the dangers of reusing single-use devices and the criteria to apply if you must reuse these kinds of devices.
The module also covered how SSDs should be laid out so that best practices in a workflow can be followed. It is crucial to train staff in standard operating procedures. Finally, you learnt about the steps for proper receipt, storage and transportation of sterile medical devices.
-
References
- Damani N. Manual of infection prevention and control. Oxford: Oxford University Press; 2019.
- Ministry of Health and Sanitation. National infection prevention and control policy. Freetown: Government of the Republic of Sierra Leone; 2015. Available from: https://afro.who.int/sites/default/files/2017-05/ipcpolicy.pdf.
- Decontamination and reprocessing of medical devices for health-care facilities. Geneva: World Health Organization. 2016.
- Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention. 2008.



