Session 3: Fundamentals of HIV Testing Services
Introduction to HTS
In this section, we will discuss HIV testing as the gateway to HIV prevention, treatment, care, and support services. We will review the Zimbabwe HTS programme, covering the types of HIV tests, different ways to reach clients to provide HTS, explain the HIV testing algorithms, and review re-testing recommendations. We will also describe commodity stock management (though we will go into more detail about stock management as part of session 15 when we talk about supply chain management).
Learning Objectives
By the end of this session, you will be able to:
- Explain the two types of HIV tests.
- Describe the self-testing and rapid HIV testing algorithms.
- Explain rapid HIV testing quality assurance and its importance.
- Highlight the importance of commodity stock management in HIV testing services.
- Describe HIV re-testing recommendations.
Learning Activities
-
Introduction to HTS (5 min)
Edwin, a 46-year-old man living in Harare, came to the clinic six years ago. He was frail and unable to walk without assistance. His health had deteriorated a great deal and he was no longer able to work. He tested positive for HIV and began treatment. His wife tested HIV positive shortly after him. They have supported each other in treatment and Edwin has gotten strong and was able to go back to work. Last year they had a baby who has tested negative for HIV. Edwin and his wife enjoy a family life together and are active in their church community.
We have heard the stories and testimonies of people whose lives have changed since finding out that they are HIV positive. They all start in with getting tested for HIV. Once people find out their positive status, with the right support and attitude they are able to maintain healthy, fulfilling lives.
HIV testing is the gateway to HIV prevention, treatment, care, and other support services. The term HIV testing services (HTS) is used to embrace the full range of services that should be provided together with HIV testing – counselling (pre-test information and post-test counselling); linkage to appropriate HIV prevention, treatment and care services and other clinical and support services; and coordination with laboratory services to support quality assurance and the delivery of correct results. The WHO 5 Cs are principles that apply to all models of HTS and in all circumstances will be discussed latter on in this session
People’s knowledge of their HIV status is crucial to the success of the HIV response. The Joint United Nations Programme on HIV/AIDS (UNAIDS) and the WHO have endorsed global goals to achieve zero new HIV infections, zero discrimination, and zero AIDS-related deaths– including the UNAIDS global fast track 90-90-90 treatment targets: By 2020, 90% of PLWHIV should know their HIV status, 90% of people LWHIV should be initiated on ART and 90% of people on ART should achieve viral suppression.
The aim of HTS is to expand coverage strategically in areas and among populations in greatest need, increase access to services, improve the quality of testing services, and help to achieve global targets. To accomplish this, there is need to assess specific situations and take into account epidemiological context and the populations most in need of HTS in different settings. It is also important to assess and, as much as possible, address social and legal barriers to access as well as issues concerning the quality of health services. Because of the potentially serious medical, social, and psychological consequences of misdiagnosis of HIV (either false-positive or false-negative), all programmes and people providing HIV testing services must strive also for zero misdiagnoses.
An HTS programme is dependent on the laboratory infrastructure and competence of HIV testing service providers. Ensuring that appropriate laboratory standards are achieved and maintained is a critical component of any HTS programme. The cost of misdiagnosis is very high and providers have an ethical obligation to ensure accurate results are given. Testing will be conducted only according to the national algorithm and adhering to the Standard Operating Procedures for the program. All HTS sites to be accredited to ensure that they meet the minimum quality standards. Testing will be conducted only by providers who are trained and competent. It is critical that service providers explain the testing procedure and possible HIV test results to the client/s before the procedure as well as conduct post-test counselling, linkages, referral, and documentation.
-
Types of HIV Tests (5 min)
Tap on each type of HIV test to read more about it.
HIV antibody
The HIV antibody test is based on the detection of antibodies to HIV (rapid diagnostic tests or ELISA).
HIV antigen
The HIV antigen tests can be DNA or RNA PCR. PCR stands for polymerase chain reaction, a method capable of detecting very small amounts of the virus in blood and tissue. The PCR is a process of amplifying minute quantities of genetic material (that is, RNA or DNA) to higher, detectable levels.
Antigen testing can detect HIV in the absence of measurable levels of antibodies (e.g., in early infection). It can also detect HIV infection in babies whose antibodies cannot be differentiated from maternal antibodies. Children born to HIV-infected mothers can carry maternal antibodies to HIV for over one year even if they do not have HIV; hence, antibody tests cannot be used to definitively diagnose HIV infection in such children.
The antigen tests become positive approximately nine days after infection, while antibody tests become positive between three and six weeks after infection.
-
HIV Testing Algorithms (10 min)
There are new HIV testing algorithms to reduce the chances of misclassification. Under the old algorithm, if the first and second tests were discordant, a third test was performed using a tie breaker test. Its results were deemed final (whether positive or negative). Since this final result wasn’t confirmed, the chances of misclassifying a client was higher.
Under the new algorithm, the steps taken to reach a positive diagnosis reduce the chances of misclassification.
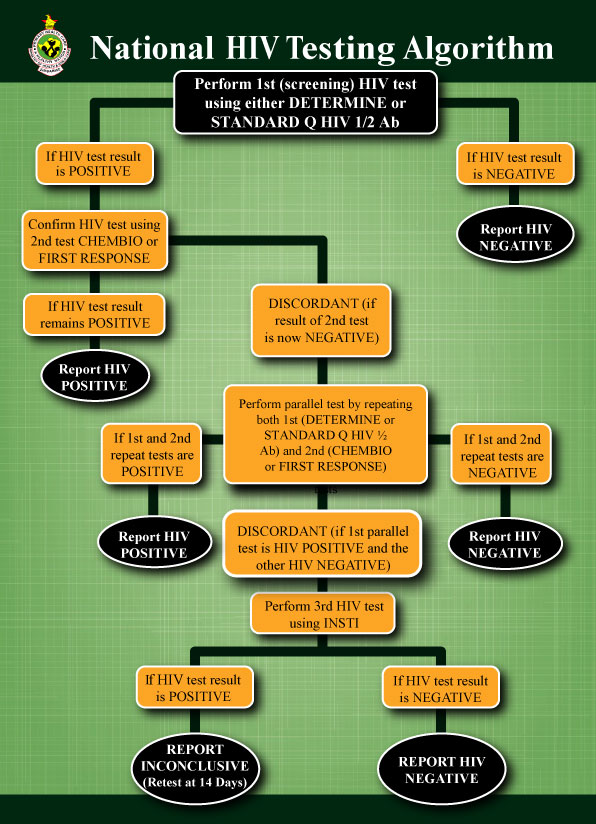
HIV Testing Algorithm
The figure below shows the national HIV testing algorithm for adults and children 18 months and above.
According to the algorithm, perform test 1 (the screening test) using Determine or Standard Q HIV ½ Ab test. If test is negative, report the result.
If test 1 is positive, perform test 2 (confirmatory test) using Chembio/ First Response. If test 2 is positive, report the test result.
If the results for both tests are discordant (that is, test 1 is positive and test 2 is negative), repeat test 1 and test 2 in parallel. If both of these tests are negative, report this result. If both tests are positive, report this result. If the two tests are discordant, perform a third test using INSTI. If this third test is negative, report as negative. If the third test is positive, report as inconclusive and retest in 14 days.
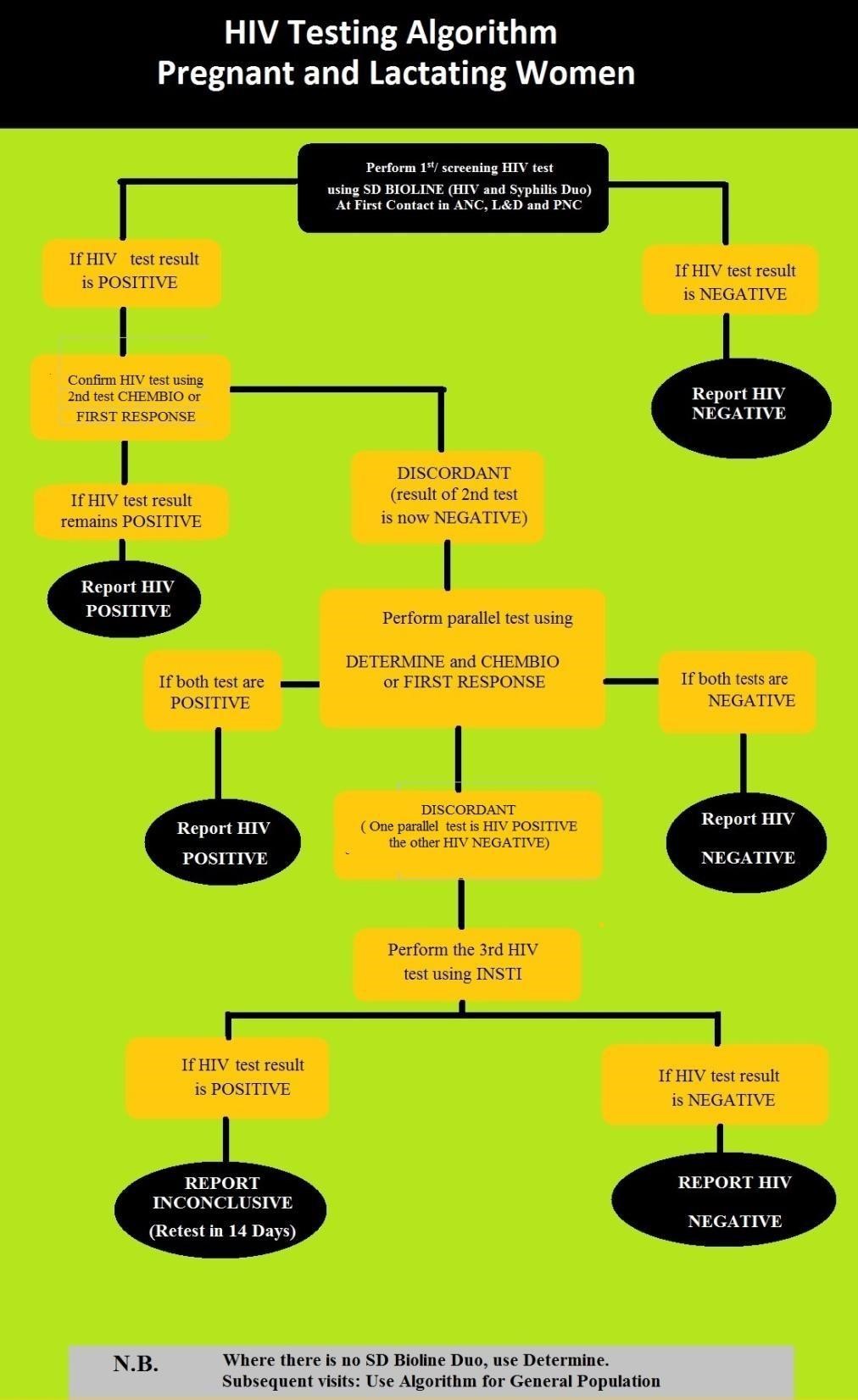
Algorithm for pregnant and lactating women
The figure below is the HIV testing algorithm for pregnant and lactating women.
-
HIV Re-Testing Recommendations (10 min)
HIV self testing is a screening test. A reactive test with self-test must be confirmed by a trained service provider using the national algorithm, starting from test 1. If test is positive client will need a retest before initiating ART. Re-testing refers to using the same testing algorithm on a second specimen from the same individual.
Re-testing to verify an HIV-positive result
Re-test all people newly and previously diagnosed with HIV before they initiate ART. Retesting should ideally be conducted by a different service provider with a different specimen. However, if there is only one health worker at the facility, they can take another blood sample a few hours apart and retest.
Re-testing people on ART is not recommended! There are potential risks of incorrect diagnosis. The effect of ART in suppressing viral replication may extend to suppression of the immune response and thus of antibody production leading to inaccurate HIV diagnosis.
Re-testing for previously HIV negative people
Most individuals do not require re-testing to verify an HIV negative status, particularly with no on-going HIV risk (e.g., re-testing to close the window period). Re-testing is needed only for HIV-negative individuals who report recent or ongoing risk of exposure. For most people who test HIV-negative, additional re-testing to rule out being in the window period is not necessary and may waste resources. This is because most people who receive HIV testing and test HIV-negative, particularly where HIV testing is offered routinely in clinical settings, will not be at risk from recent infection.
Retesting during the window period: The post-test counselling messages recommend that all people who test HIV negative should return after three months to rule out acute infection that is too early for the test to detect (window period). Additional re-testing to rule out being in the widow period is no longer necessary except for HIV-negative individuals who report recent or ongoing risk of exposure
Recommendations
This table below shows when to re-test for each group of people.
Population Recommendation General population at on-going risk behaviours including people with a known HIV-positive partner, individuals seen for diagnosis or treatment of STIs, people with known recent HIV exposure, TB patients who are at high risk of HIV exposure, OPD patients with OIs, individuals taking PEP and PrEP Offer re-testing at least annually Individuals on PrEP Re-test after every three months Key Populations Re-testing according to risk assessments (suggest three monthly) HIV-negative pregnant women and lactating women Re-test previously HIV-negative women in the first trimester of pregnancy and at third trimester/ or at delivery; six weeks post-natal, and six monthly during the breastfeeding period. Visits to EPI and six weeks (DTP) and at nine months (measles) should be time points where maternal HIV status is reassessed. HIV-positive individuals before initiation of ART Re-test all people newly and previously diagnosed with HIV before they initiate ART. Re-testing should ideally be conducted by a different service provider with a different specimen. However, if there is only one health worker at the facility they can take another blood sample a few hours apart and retest. Health workers should retest previously HIV-negative pregnant and lactating women as follows in order to diagnose infection early and initiate life-long ART to prevent mother to child transmission of HIV. This table shows when to re-test for HIV-negative pregnant and lactating women.
Re-testing HIV-negative pregnant and lactating Women
Time of Presentation When to Test First or second trimester: HIV-negative status known Re-test in third trimester First or secon trimester: HIV-negative status unknown Offer HIV test; re-test in third trimester Third trimester: HIV-negative status known Re-test 6 weeks post delivery Third trimester: HIV-negative status known Offer HIV test; re-test at 6 weeks post delivery Labour and delivery: HIV-negative status known from third trimester Re-test 6 weeks post delivery Labour and delivery: HIV-negative status unknown from first or second trimester Re-test immediately, then 6 monthly Labour and delivery: HIV-negative status unknown Offer HIV test, then 6 monthly Breastfeeding woman: HIV-negative status known Re-test every 6 months until cessation of breastfeeding Breastfeeding woman: HIV-negative status unknown Offer HIV test immediately.
Retest every 6 months until cessation of breastfeeding -
Quality Assurance for Rapid HIV Testing (5 min)
In the context of HIV testing services, the Quality Assurance Programme is the overall programme that ensures the final HIV test results reported are correct. A false result may irrevocably damage the reputation of the HTS programme and cause untold suffering to the client. Correct HIV test result is a priority and a crucial component of WHO’s 5 Cs for HTS.
Quality assurance for HTS is implemented through quality management systems comprising of internal quality control and external quality assessment. Tap on each system below to learn more.
Internal quality control includes:
- Ensuring good laboratory practices with set standards for performing HIV tests.
- Checking rapid HIV test kits storage and expiry dates.
- Running positive and negative samples daily before testing clients, when opening a new kit, and on receiving a new batch.
- Providing support, supervision, and mentorship.
- Accurately documenting and reporting at all entry points and consolidating data.
External quality assessment includes:
- Enrolling all testing sites with an external quality assurance programme (APHL or ZINQAP) to participate in EQA.
- Ensuring that each facility/tester should receive dried tube specimens (DTS) for proficiency testing (pt) at regular intervals. Root cause analysis and corrective action is provided by the laboratory team for facilities/testers that do not perform well.
-
Laboratory Safety Rules (5 min)
It is important for testers to follow strict laboratory safety precautions based on recommendations adopted by the National Microbiology Reference Laboratory. Each facility must have a site-appropriate guide on laboratory safety precautions.
All staff are to take precautionary measures to protect against blood contamination.
In case of an accidental exposure during testing, follow the national PEP policy, as covered in Session 1 on Prevention.
-
Stock Management (5 min)
Every facility providing HTS will have staff members trained in integrated stock management.
All commodities upon receipt should:
- Be counted and crosschecked with delivery documentation.
- Stored in well-lit and ventilated rooms.
- Stored to allow first expiry first out.
Every HTS entry point should maintain stock cards for each test kit, ensure adequate stocks, and order in accordance with the MOHCC supply chain system. No commodity should expire in stock, so ensure use or redistribute before expiry date. For proper storage of test kits and DNA PCR bundles, follow the manufacturer’s instructions.
Note that this will be covered in more detail during Session 15 on Supply Chain Management.
-
Knowledge Check (10 min)
-
Key Points (5 min)
- HIV antibody tests (ELISA and rapid tests) become positive three to six weeks after a person is infected.
- HIV antigen tests (PCR) detect virus in the blood and are positive nine days after infection. These are effective during the window period.
- Retesting refers to using the same testing algorithm on a second specimen from the same individual. Retesting during the window period is no longer necessary except for clients at on going risk of HIV infection and treatment requirements.
- All newly and previously diagnosed clients must be retested to verify HIV status before ART initiation.
- The cost of misdiagnosis is very high, and providers have an ethical obligation to ensure accurate results are given.
- Every facility providing HTS needs to ensure adequate potent commodity stocks, stock cards for each test kit at all entry points and order in accordance with the MOHCC supply chain system.