Session 12: ART in Pregnant and Breastfeeding Women
Clinical Assessment
In this section we will discuss the clinical assessment for HIV-positive pregnant and breastfeeding women. We will then discuss ART regimens and their side effects related to PMTCT. We will learn how to provide prophylaxis for pregnant and breastfeeding women and their children. We will also discuss how to identify the different levels of risk of MTCT.
Learning Objectives
By the end of this section, you will be able to:
- Describe the laboratory investigations for HIV-infected pregnant and breastfeeding women.
- Adhere to special considerations during pregnancy: appropriate ART prescription; monitoring for and managing side effects and toxicities.
- Describe OI and malaria prophylaxis for HIV-infected pregnant and breastfeeding women and prophylaxis for HIV-exposed infants.
- Describe the levels of risk of MTCT.
Learning Activities
-
Clinical Assessment of HIV-infected Women (5 min)
Sekai is 22 years old and has come in for her first ANC visit. She is four months pregnant and has just been diagnosed HIV positive. We know we need to pay some special attention to Sekai to keep her healthy throughout the pregnancy and birth. We also know that we can help her to have a healthy baby who is HIV-negative and stays negative. We are going to review the clinical care components that need to be addressed during her clinic visit.
Although clinical and immunological assessments remain integral in caring for HIV-infected pregnant women, they are not prerequisites for commencement of lifelong ART in this population. Assessment of HIV-infected pregnant women consists of routine pregnancy-related care as provided to all pregnant women, plus HIV clinical, immunological, and virological assessment and review.
You should perform a clinical assessment of the HIV‐infected pregnant woman every time she comes for antenatal services, delivery, and for postnatal follow‐up. The criteria for WHO clinical staging for pregnant woman are the same as for any other adult. The only modification is the weight loss criteria in clinical stages 2 and 3. For a pregnant woman failure to gain weight during pregnancy may be considered as weight loss.
Routine clinical staging should be conducted for all pregnant women. Their CD4 count and viral load will guide the assessment of disease progression even though those test results do not determine eligibility for ART.
-
Lifelong ART (Option B+) (5 min)
Lifelong antiretroviral treatment to HIV-positive pregnant or lactating women, regardless of their CD4 count or clinical stage.
Lifelong administration of a combination of a minimum of three ARVs to a pregnant or lactating woman is used for the treatment of maternal HIV infection and prevention of MTCT of HIV. ART during pregnancy and lactation will improve the health of the woman and is the most effective intervention for decreasing the risk of transmission of HIV to the infant.
However, treatment decisions for pregnant women should be based on:
- Co‐existence of other medical conditions
- Whether the woman is on any other medication
- Potential side effects of ART in pregnant women
HIV care services should be integrated into routine antepartum care within maternal, neonatal, and child health (MNCH) settings. In the case of HIV-positive pregnant women already on ART, mothers are transferred from the general OI/ART clinic for continued HIV care/ART in the MNCH up to 24 months after delivery. Ideally all HIV-infected male partners should also receive HIV care and treatment within the MNCH department for the 24 months.
-
Laboratory Investigations (5 min)
Tap on the boxes below for more information about each of the laboratory investigations.
CD4 count
ALL HIV-infected pregnant and breastfeeding individuals are eligible for lifelong ART. The absence of a CD4 count should not delay ART for any pregnant or breastfeeding woman but should be done at baseline to determine if there are additional medications needed to prevent infection such as PCP.
Renal function
Assessment of renal function is important, since the preferred regimen contains tenofovir, which might cause renal toxicity and is contraindicated in renal failure.
- A test to check renal function (urine dipstick and/or creatinine level) is recommended before initiating ART.
- In the absence of the tests, history taking on urinary symptoms (oliguria, polyuria, haematuria, frequency) and clinical examination for signs of renal failure assist in guiding the clinician.
- It is important to note that the absence of the test for renal function should not delay ART initiation in pregnant and breastfeeding women.
Haemoglobin
This should be done as part of the routine care in ANC in all pregnant women. If the haemoglobinometer is not available onsite, clinical examination for pallor will guide the clinician.
The preferred ART regimen in pregnant women (TDF/3TC/EFV 600) has not been shown to cause or worsen anaemia, and the absence of Hb count should not delay ART initiation on this regimen.
If the alternative AZT-containing regimen is prescribed, then it is important to check the Hb level before initiation of AZT. Monitor Hb again at two weeks, four weeks, and then every three months, as AZT may cause or worsen pre-existing anaemia.
Viral load testing
In terms of mother-to-child transmission, the higher the viral load (VL) of the mother, the higher the risk of transmission to the baby. For this reason, HIV-positive pregnant and breastfeeding women should be prioritised for early initiation on ART and for viral load monitoring.
-
Viral Load (VL) Monitoring (5 min)
The algorithm for HIV-positive pregnant women and breastfeeding mothers is different than the one for the general population infected with HIV. This algorithm further divides this special population into women already undergoing treatment and women who are newly diagnosed or newly on treatment.
Alogrithm for VL Monitoring for Pregnant Women on ART
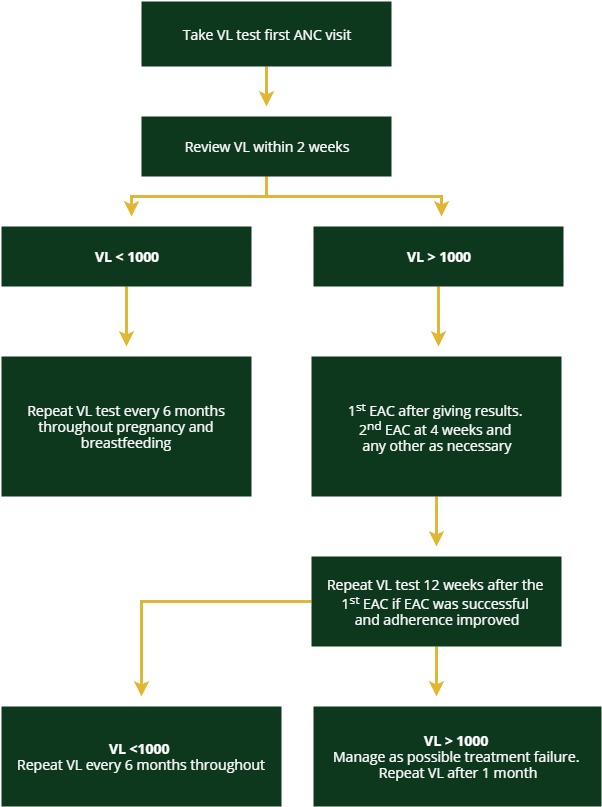
Algorithm for VL Monitoring
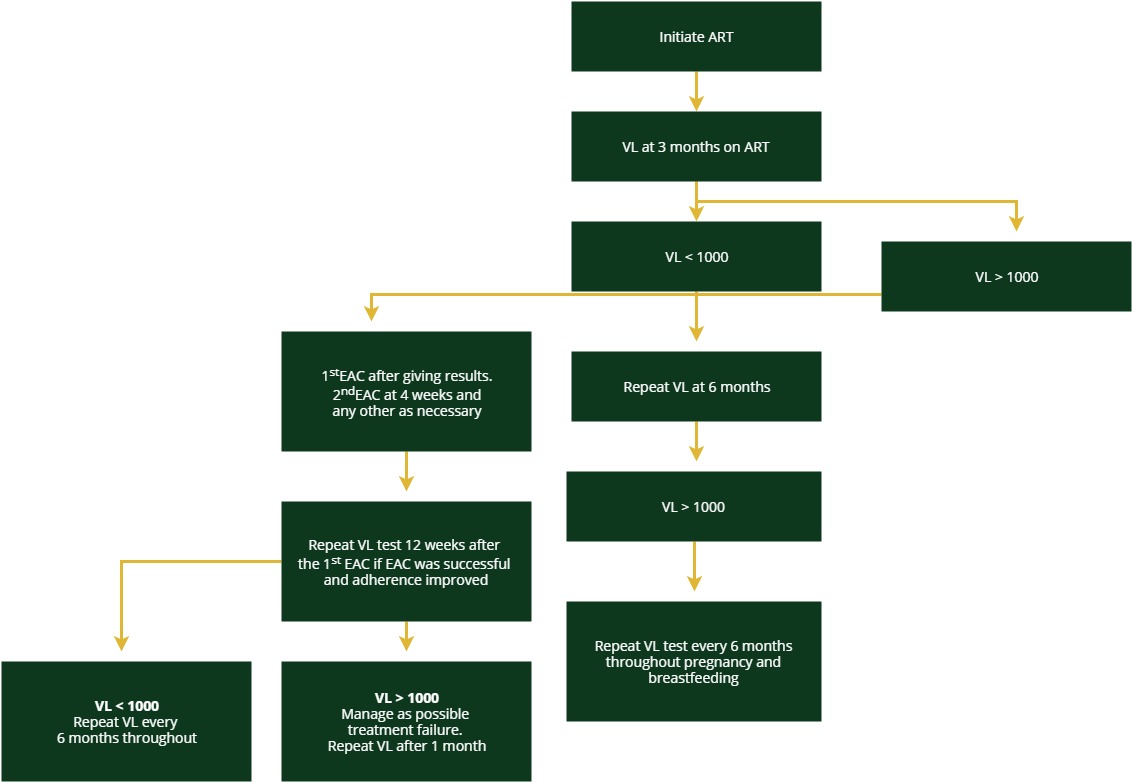
Pregnant women on ART
Pregnant women on ART should receive a VL test during their first ANC visit. The viral load should then be reviewed within two weeks.
Pregnant women not on ART
Pregnant women who test positive should get a confirmatory test and start ART immediately. After three months on ART, conduct a VL test.
The rest of the algorithm is the same for each group. A viral load test and results taken in the third trimester (32 weeks of gestational age and/or later will be useful for risk stratification then VL monitoring will be done every six months during the breastfeeding period. Tap on each category to see how to manage clients with high or low VL.
Low VL (<1000 copies/mL)
If the client has a low VL (‹1000 copies/mL), then the test should be repeated every six months throughout the remainder of pregnancy and breastfeeding.
High VL (>1000 copies/mL)
If the client has a high VL (>1000 copies/mL), then enhanced adherence counselling (EAC) should be offered after giving the results to the client. Because the goal is to prevent mother-to-child transmission, for pregnant and breastfeeding women we need to assume drug resistance is the cause for high VL earlier than we would for the general population. Therefore, if the first VL test result shows a high VL, we would assume drug resistance has developed and would switch the client to second-line therapy (in consultation with your clinical mentor) if the repeat VL test after one month of EAC does not improve.
-
Tariro’s Clinical Assessment (10 min)
Tariro is a 26-year-old woman who is being seen in your clinic for her first antenatal visit at 24 weeks. She agreed to an HIV test today even though she tells you that she tested negative when she was evaluated for the STI a year ago. Tariro’s HIV test today is positive. You provide thorough post-test counselling and offer support since Tariro is shocked and upset at the results.
Your assessment reveals that her past medical history includes treatment for an STI at another clinic; otherwise it’s unremarkable and she isn’t currently on any medication. Tariro has had no surgeries in the past. She has no current complaints and has felt fine throughout her pregnancy after an initial few weeks of mild nausea. She tells you that her appetite really didn’t return to what it used to be before she was pregnant but that she has gained weight.
Tariro’s physical exam results are
- HEENT – WNL: No thrush noted
- Chest: lungs clear to auscultation
- Breasts: no abnormalities noted. Skin discolored and scared on right side of chest below the clavicle continuing to the mid-axilla area. Tariro tells you that this is a result of the rash she had.
- Musculoskeletal: no LE edema noted
- Foetal heart rate: 132 beats per minute
- MUAC: 24
-
ART Regimens in PMTCT (5 min)
Sekai, the 22-year-old who is four months pregnant and has just been diagnosed HIV-positive at her first ANC visit, is now ready for ART. You have done a thorough clinical examination and prepared her for treatment. (We will go into more detail in the next section about counselling). Sekai has accepted her diagnosis and is committed to making sure both she and her baby stay healthy.

Once you have completed your clinical assessment of your HIV-positive pregnant client, and you have prepared her for treatment, you will need to initiate ART at this visit. Remember, newly diagnosed HIV-positive pregnant women should be counselled and initiated on ART on the day of diagnosis.
The preferred first line ART regimen for pregnant and breastfeeding women is the single-formulation, one tablet, once-a-day fixed-dose combination of TDF/3TC/EFV 600. The table below lists the ART regimens for the mother.
First-Line ART Regimen Alternate First-Line Regimen TDF + 3TC + EFV (600 mg)
(Triple fixed dose)
- TDF + 3TC + NVP*
- AZT + 3TC + EFV 600 or NVP
Efficacy of low-dose EFV in pregnancy has not been studied
DTG has not been sufficiently studied in pregnant women
*Avoid use of NVP if CD4 above 250
-
Encouraging Positive Living (10 min)
In addition to ART, you should emphasise the need for other “positive living” strategies and behaviour changes.
-
Special Considerations & Monitoring During Pregnancy (15 min)
There are special considerations and precautions that you should be aware of when administering ART during pregnancy. In HIV disease, the benefits of ART outweigh the potential risk to the foetus. Your pregnant client must be assisted to understand the risks and benefits of ART, focusing on the benefits! If she is already on ART when she becomes pregnant, ART should be continued; the dosage is the same as for non-pregnant adults and adolescents. If she is on EFV, and is in the first trimester, continue with EFV as it is no longer contraindicated.
There are three key conditions that should be routinely monitored during pregnancy.
Tap on the boxes below to read more about each one:
Nevirapine-related rash/liver toxicity
There is an increased risk of severe nevirapinerelated rash and liver toxicity if it is given to pregnant woman with CD4 count more than 250. If a Nevirapine-containing regimen is used, the woman should be carefully monitored for clinical signs and symptoms of liver toxicity, and, if available, an ALT should be done at baseline and closely monitored at two-four weeks, and again at three months, after initiation of nevirapine. Liver toxicity is most common in the first month but can occur at any time after the first month.
Anaemia
Anaemia is a common problem in pregnancy, due to poor iron stores and infection, such as malaria or hookworm. Additionally, HIV infection itself, cotrimoxazole and AZT can contribute to anaemia. AZT, in particular, can cause a very rapid fall in haemoglobin.
If the woman’s haemoglobin is less than 7 g/dl:
- Do not start AZT.
- Determine the cause of the anaemia and treat appropriately.
- If the woman is already on AZT prophylaxis and she develops severe anaemia, stop AZT.
- For pregnant women with persistent severe anaemia (Hb‹7 g/dl) in whom AZT is contraindicated, use another triple ARV regimen.
If the woman is already on an AZT-based ART regimen, stop AZT and substitute with TDF.
Tenofovir renal toxicity
It is rare for tenofovir to cause renal toxicity in otherwise normal kidneys, but common for it to aggravate pre-existing renal impairment; hence, TDF should not be started in clients with known pre-existing renal conditions. Renal assessment using urinary signs and symptoms and urine dipstick is done at baseline.
New Signs and Symptoms in a Pregnant or Postpartum Woman on ART
There are a number of reasons that you may see new signs or symptoms in pregnant or postpartum women. Any of the following reasons may be the cause:
- Pregnancy-related problem or complication
- A side effect of the ARV or any other drugs
- New opportunistic infections (OIs)
- Immune reconstitution inflammatory syndrome (IRIS)
- A common infection or problem that is not directly related to HIV or pregnancy (e.g., malaria)
The challenge we face in caring for these women is to correctly recognise the signs and symptoms, decide what may be the cause and provide the appropriate care in a timely manner.
Side Effects of ARVs
Pregnant women on ART experience some side effects that are similar to people taking ART who are not pregnant. Most side effects are minor; they include nausea, fatigue, and diarrhoea. In rare instances, however, they can also be very serious.
The side effects of ARV medicines that are most likely to be confused, or even combined with, pregnancy-related problems or complications are nausea and vomiting, headache, fatigue, pallor/anaemia, fever, jaundice, abdominal/flank pain, cough/difficult breathing, and depression.
Tap on each sign or symptom to learn how to manage it.
Sign or symptom Response Nausea, vomiting Take the medicine with food. If she is on AZT, reassure the client that this is common and usually self- limited. Treat client symptomatically.
If it persists more than 2 weeks or worsens, call for advice or refer.
Additional considerations: This is a common pregnancyrelated problem in the first 14 weeks of gestation. Morning sickness can be made worse by nausea from ARV medicine. If no response to dietary changes and no other signs or symptoms, try vitamin B6 (25mg 3-4 times daily, not to exceed 100mg/day). If no response and vomiting is interfering with ART or fluid intake, give diazepam rectally. If no response, consult or refer.
Headache Assess for meningitis.
Additional considerations: Measure BP. If diastolic BP >90 mm Hg, consider pre-eclampsia. Take a blood slide for malaria parasites or rapid diagnostic test (RDT) if malaria is suspected.
If on AZT or EFV, reassure that this is common and usually self-limited. If it persists more than two weeks or worsens, consult or refer. Give paracetamol if no underlying problem.
Diarrhoea Prevent dehydration and when it happens, hydrate. Follow diarrhoea guidelines in IMAI Acute Care guideline module. Reassure client that, if due to ARV, it will improve in a few weeks. Follow up in two weeks and if it has not improved, call for advice or refer. Fatigue Consider anaemia, especially if on AZT. Check for pallor and check haemoglobin. If fatigue is due to anaemia and the Hb level is ‹7 g/dl, stop AZT or replace AZT with TDF if on ART. Manage the anaemia.
If not because of anaemia, fatigue commonly occurs when starting AZT and lasts four to six weeks. If severe or longer than this, consult or refer.
Additional consideration: Fatigue is common in pregnancy. Rule out other causes. If the woman’s haemoglobin is ‹11 g/dl.
Pallor: anaemia Measure haemoglobin. If severe pallor or pallor with other signs of severe anaemia or very low haemoglobin (‹7 g/dl), stop AZT and refer/consult with a clinician; substitute TDF for AZT if on ART.
If anaemia not severe, refer to IMAI Acute Care guideline module, p. 18. Make sure any client with anaemia (Hb‹11 g/dl) is given iron/folate tablets, mebendazole tablets (if not given in the last six months), and malaria treatment, if at risk.
Anxiety, nightmares, depression, suicidal ideas This may be due to efavirenz. Give EFV in the evening before bed; counsel and support (usually lasts less than three weeks).
Call for advice or refer if severely depressed, psychotic, or suicidal. Initial difficult time can be managed with amitriptyline at bedtime.
Additional considerations: Consider depression during pregnancy and postpartum depression in the first weeks after birth.
Blue/black nails Reassure, no danger. Common with AZT. Rash If on nevirapine, abacavir, or cotrimoxazole, assess carefully. Call for advice.
If generalized or peeling or with mucous membrane involvement, stop all the ARV medicine and cotrimoxazole and refer to hospital (suspect SJS). Additional considerations: If pregnant woman is on nevirapine, a new rash is likely due to this and may indicate a life threatening situation. Pregnancy-related rashes are rare, and this diagnosis is made clinically. Any pregnant woman with unrelenting pruritis should be evaluated by the doctor urgently.
Fever Check for common causes of fever (Use IMAI Acute Care guideline module). Consult or refer. Fever could be due to side effect of the ARV medicine, an opportunistic or other new infection, or immune reconstitution syndrome.
Additional considerations: Consider a pregnancy or postpartum related infection.
Yellow eyes (jaundice) Stop all medications. Call for advice or refer if abdominal pain is also present (abdominal pain might be due to pancreatitis from DDI or D4T).
If jaundice or liver tenderness, check alanine aminotransferase (ALT) test.
Nevirapine is most common cause. Consult or refer.
Additional considerations: Consider a pregnancy or postpartum related problem. Jaundice in pregnancy can be caused by many diseases, some of which can be fatal if not managed urgently and correctly. All pregnant women with jaundice should have an urgent evaluation by a doctor and a liver function test done.
Abdominal or flank pain Additional considerations: Abdominal or flank pain: consider abruption placenta, labour, and conditions more common in pregnant women, such as pyelonephritis. Consult or refer. Tingling, numb, or painful feet/legs or hands If new or worse on treatment, consult or refer. Client on D4T + 3TC + NVP should have the D4T discontinued; substitute D4T with TDF. Cough or difficult breathing This could be an opportunistic infection or immune reconstitution inflammatory syndrome (IRIS) or a side effect of the ARV medicines (i.e., lactic acidosis). Call for advice. (If lactic acidosis or IRIS is suspected, stop drug and consult/refer). See IMAI Acute Care guideline module section on cough or difficult breathing.
Additional considerations: Consider severe anaemia, cardiac failure, respiratory infection, and pulmonary embolus.
Changes in fat distribution This can lead to poor treatment adherence. Discuss carefully with your client:
- Can she/he accept it? Counsel and educate the client.
- Consider substituting D4T or AZT with TDF.
Signs of kidney problems in clients on tenofovir Refer if suspected kidney problem. If possible, determine serum urea and creatinine levels. If abnormal, consult or refer. -
Check Your Knowledge (5 min)
-
Netsai (5 min)
Netsai is a 21-year-old woman who has come to your health facility for an antenatal visit. She is about 38 weeks pregnant and 2 weeks ago was started on TDF-3TC-EFV. At the previous visit her haemoglobin was 10 g/dl, and the nurse told her to take one iron tablet daily. Today, she is complaining of fatigue and diarrhoea. The diarrhoea does not have any blood in it, and she has no fever or any other accompanying signs or symptoms. After you have examined her, you see that she has not gained weight since the last visit and has palmer and conjunctival pallor. Her vital signs are: BP 120/78, pulse 78 beats per minute, respirations 18 per minute, temperature 37°C. Her CD4 count, done 2 weeks ago, is 540.
-
OI & Malaria Prophylaxis (5 min)
We met Tariro earlier, she is a 26-year-old woman who is being seen in your clinic for her first antenatal visit at 24 weeks. Your assessment reveals that her past medical history includes treatment for an STI at another clinic; otherwise her history is unremarkable and she isn’t currently on any medication. Tariro has had no surgeries in the past. She has no current complaints and has felt fine throughout her pregnancy after an initial few weeks of mild nausea. You determine that her rash was suspected herpes zoster and that she is in WHO clinical stage 2. You realize that she is eligible for prophylaxis against PCP and toxoplasmosis.
Tap each tab to read how to provide prophylaxis for pregnant and breastfeeding women.
Cotrimoxazole preventive therapy (CPT)
All HIV-positive pregnant women in WHO clinical stage 2, 3, or 4 should be started on cotrimoxazole preventive therapy for prophylaxis against PCP, toxoplasmosis, and if needed for chronic diarrhoea. Dosing is as follows:
- Cotrimoxazole (CMZ) 960 mg daily should be started 2 weeks after ART initiation in pregnant women who have been newly diagnosed of HIV and initiated on ART.
- Those with CD4 count greater than 350 and are on lifelong ART need not be given CPT unless clinically indicated.
- In settings where malaria/ or severe bacterial infections are prevalent; provide cotrimoxazole prophylaxis to pregnant and breast-feeding women regardless of CD4 cell count and WHO clinical stage
Isoniazid preventive therapy (IPT)
All HIV-positive pregnant women should be screened for TB using the TB screening tool at every ANC visit. Presumptive TB patients should submit one sputum specimen for Gene Xpert. If positive/RIF resistance not detected, they should be started on TB treatment immediately. If your pregnant client has had active TB excluded, she should be initiated on Isoniazid 300 mg daily and pyridoxine 25 mg daily for six months.
Continue to screen for TB regularly in your HIV-positive pregnant clients at subsequent visits to the facility.
If your pregnant HIV-positive client is on IPT, assess her for side effects (e.g., rash, nausea and vomiting, severe fatigue, loss of appetite, numbness). MCS is used for treatment follow-up.
Malaria prophylaxis (IPTp)
Intermittent Preventive Therapy in pregnancy (IPTp) with sulfadoxine and pyrimethamine (SP) is given to pregnant women in malaria prone areas.
You should give four doses of SP given 4 weeks apart starting after 16 weeks of gestation. All HIV-positive pregnant women on cotrimoxazole should not be given SP (IPTp).
-
HIV-exposed Infants (HEI) ARV Prophylaxis (5 min)
All newborns exposed to HIV should receive ARV drugs to reduce the risk of transmission. For a newborn, you should start ARV prophylaxis as soon as possible after birth. Duration depends on the breastfeeding status and on the infant’s risk status.
When we talk about high MTCT risk, the criteria is as follows:
- Maternal VL over 1000 copies/mL at > 32 weeks gestation
- Newly diagnosed HIV-positive mothers during labour and delivery and breastfeeding period
- Incident infection (sero-conversion) during pregnancy and breastfeeding
- No ART or ‹ 8 weeks of ART at delivery
All infants who do not meet the criteria for ‘high-risk’ infants are classified as ‘low-risk’ infants.
Dosing
This table shows the dosing for AZT and NVP infant prophylaxis.
Infant NVP and AZT Dosing Schedule
Infant Age NVP Dosing AZT Dosing Birth to 6 weeks Birth weight ‹2000gm and >35 weeks of GA* 2 mg/kg per dose o.d. 4 mg/kg per dos b.d. Birth weight 2000-2499gm 10 mg o.d. (1 mL of syrup) 10 mg b.d. (1 mL of syrup) Birth weight ≥2500gm 15 mg o.d. (1.5 mL of syrup) 15 mg b.d. (1.5 mL of syrup) 6-12 weeks 10 mg o.d. (2 mL of syrup o.d. OR half a 50 mg tablet o.d.) 60 mg b.d. (6 mL of syrum b.d. OR a 60 mg tablet b.d.) Cotrimoxazole prophylaxis
You should start cotrimoxazole prophylaxis in all HIV exposed infants from 6 weeks of age until HIV infection has been ruled out at the end of the mother-to-child transmission risk period. For infants who have confirmed HIV infection, the cotrimoxazole prophylaxis should be continued though childhood and adolescence. For high risk HEI the cotrimoxazole should be taken concurrently with the NVP+AZT prophylaxis (NVP +AZT prophylaxis should be given as early as possible from birth. The duration will depend on whether the infant is high or low risk, breast or formula fed).
This table shows the formulation and dosage for cotrimoxazole for HIV-exposed and infected children.
Recommended Daily Dosage Suspension (5 mL syrup, 200 mg/40 mg) Pediatric tablet 100 mg/20 mg Single strength adult tablet (400 mg/80 mg) ‹6 months (100 mg/20 mg) Once daily 2.5 mL One tablet ¼ tablet, possibly mixed with feeding 6 months – 5 years (200 mg/40 mg) Once daily 5 mL Two tablets Half tablet -
Tariro’s Treatment Plan (10 min)
We met Tariro earlier, a 26-year-old woman being seen in your clinic for antenatal care. Your assessment reveals that her past medical history includes treatment for an STI at another clinic; otherwise, her history is unremarkable and she isn’t currently on any medication.
Tariro has had no surgeries in the past. She has no current complaints and has felt fine throughout her pregnancy after an initial few weeks of mild nausea. You had determined that the rash she had was suspected herpes zoster and determined she is in WHO clinical stage 2.
Tariro has been adherent to her medications and has taken her cotrimoxazole as directed. Her VL has come back as target not detected (TND).
-
Key Points (5 min)
- Assessment of HIV-infected pregnant women consists of routine pregnancy-related care plus HIV clinical, immunological, and virological assessment and review.
- Clinical assessment of an HIV-infected pregnant woman should be performed every time she comes in for services.
- Laboratory investigations for pregnant women should include renal function and haemoglibin testing.
- Pregnant women should be prioritized for viral load testing.
- The preferred first line ART regimen for pregnant and breastfeeding women has is the single-formulation, one tablet, once-a-day fixed drug combination of TDF/3TC/EFV.
- Side effects of ARVs are likely to be confused or combined with pregnancy-related problems.
- Prophylaxis for OIs and Malaria should be provided as directed in Zimbabwe guidelines.
- All newborns exposed to HIV should receive ARV drugs to reduce the risk of transmission, dosing for AZT and NVP should follow the MoHCC dosing schedule.
- Cotrimoxazole prophylaxis should be started in all HIV exposed infants from six weeks of age until HIV infection has been ruled out.
