Oyster Drills |
 Ocinebrellus with eggs |
 Urosalpinx with tag |
Introduction:Charles Elton, the great animal ecologist, wrote in 1958: “The greatest agency of all that spreads marine animals to new corners of the world must be the business of oyster culture.” Elton was referring in part to two oyster drills that had already arrived in Washington with imported oysters. Oysters were transported from the US east coast (late 1800s-1913) and from Japan (1919-1977) as small individuals that were grown to market size on Washington tideflats. There are records of nearly a half-billion kilograms of shell arriving on the west coast from Japan alone, and this shell served as a vector for numerous introduced species. According to Wonham and Carlton (2005 Biological Invasions), oyster imports have been the single largest vector on the west coast: 20% of all non-native species are known to have arrived with shipments of commercial oysters. Oyster drills are muricid snails that eat oysters, other bivalves, and barnacles. Even before their arrival, shellfish growers suspected that they would be pests, and attempts were made to prevent their entry. For instance, oysters were inspected by industry or Washington state biologists prior to packing in Japan. Nevertheless, the Eastern drill (Urosalpinx cinerea) established in Willapa Bay and in Boundary Bay around the turn of the 20th century, and the Asian or Japanese drill (Ocinebrellus inornatus) established in Puget Sound by 1935 and in Willapa Bay by 1965. Since that time, Washington regulations have prohibited the transfer of oysters or shell from infested to clear areas, and this has probably slowed the spread of drills, since their life cycle does not include a planktonic larval phase. |
|
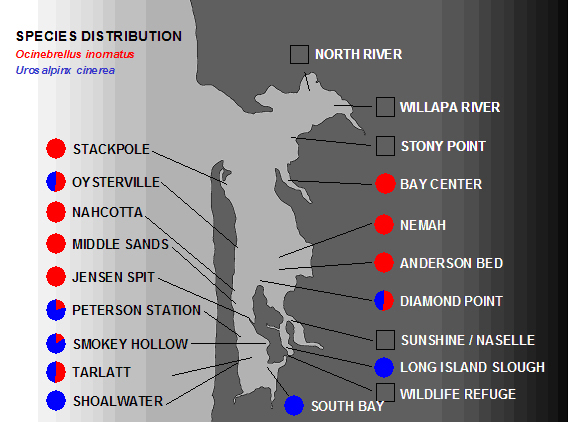 This figure of the modern distribution of the two oyster drills is based on collections of thousands of individuals throughout Willapa Bay. Empty squares refer to locations where no drills were discovered despite several person-hours of searching among oyster shells. |
|
Impacts:Oyster drills are an economic pest. They may also be partly responsible for the failure of native oysters to recover from overexploitation. We have documented that oyster drills of both species prefer small ( less than 2 cm) oysters and can consume up to 3 per week. |
|
Demography:We have been studying the demography of these two species of oyster drills through a two-year grant from NOAA/Sea Grant. Mark-recapture studies of 2516 Urosalpinx and 2629 Ocinebrellus were carried out from April 2002-April 2004 at three sites in Willapa Bay, WA. Each time we captured an individual, we gave it a unique tag (color-coded number on a vinyl tag, super-glued to the shell) and measured shell length (nearest 0.1 mm). Only once have we observed an individual marked in one experimental area to move to another only meters apart. Tag loss has been minimal: less than 1% of double-marked individuals have shown evidence of tag loss, apparently due to failed predation events. |
|
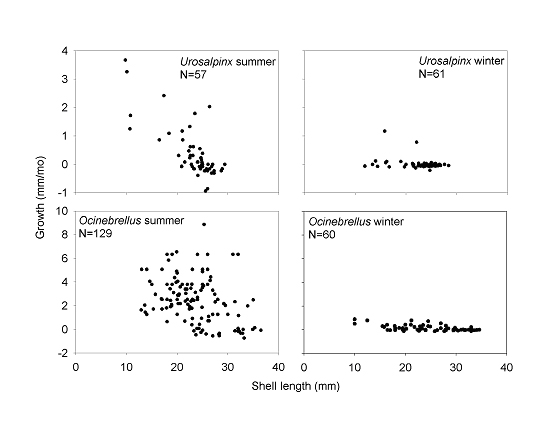 Juvenile oyster drills grew rapidly, with shell growth rates declining with size. These growth rates suggest that drills reach reproductive size in 1-2 years. In laboratory studies, we have not observed egg capsules to be laid by Ocinebrellus less than 30 mm or by Urosalpinx less than 23 mm, and these lengths require 1-2 years to achieve. Adult survival rates were low, less than 10% annually for Ocinebrellus and less than 30% annually for Urosalpinx (80-93% monthly). These low survival rates surprised us, and we now consider Ocinebrellus to have a “live fast, die young” life history, apparently compensated by high fecundity. |
|
Control:Ad hoc control efforts have not successfully eradicated or reduced the distribution of oyster drills. Oyster growers who are concerned about mortality from drills generally practice manual control: they go out on the tideflat with 5 gallon buckets and pick up as many drills as they can. Advice on effective control strategies would be extremely beneficial to shellfish growers adjusting to impacts of these pests and to state biologists charged with managing them. We built stage-structured population models to explore the consequences of control at different phases of the life cycle, particularly comparing the effectiveness of removing adults or removing egg capsules. |
|
Egg capsules are bright yellow, larger than rice grains, and laid in clusters usually from April to July. Our modeling results indicate that the removal of egg capsules is more effective at reducing population growth than is the removal of adults (although removal of both is obviously even better). This result can be generalized to a “rule of thumb” that, for species that are increasing in abundance, the effectiveness of removing the adult phase is directly related to adult survival. Because adults of Ocinebrellus in Willapa Bay have relatively low survival, their removal does not help much to reduce population growth. |
|
UW Biology | University of Washington |
Created by Lee McCoy, Updated by Jerome Tichenor, March 19, 2013 |