KEN SHE Study on HPV-vaccine Efficacy
Study Design
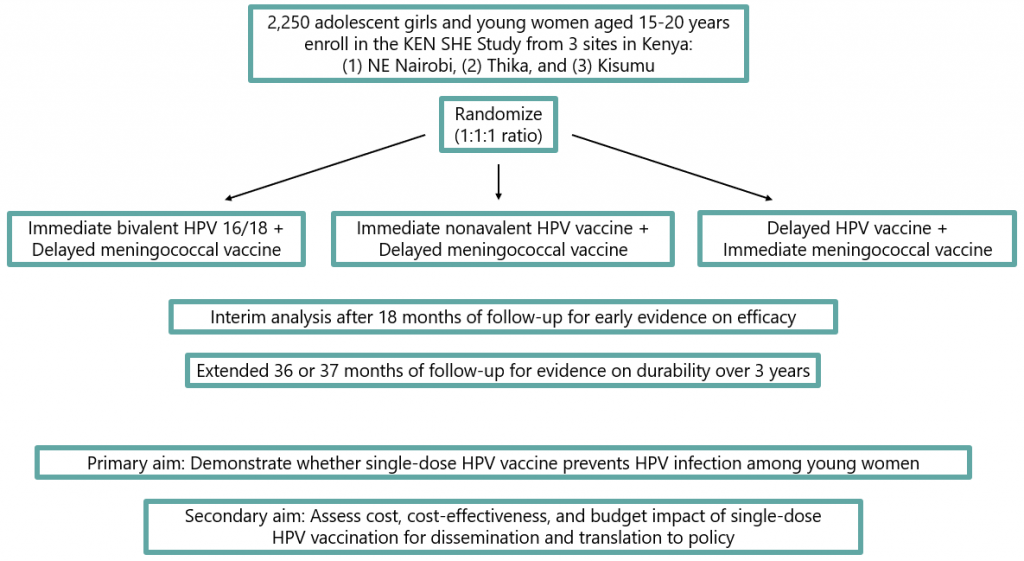
Each woman aged 15-20 years who enrolls in The KEN SHE Study will be randomly assigned to either receive immediate HPV vaccination or immediate meningitis vaccination. She will then be asked to return for clinic visits every 3 months for 3 years. During these visits, she will receive health checks and be tested for HPV infection. At the end of the study, the woman will be given the vaccine type she did not receive at study start. If the World Health Organization recommends multiple HPV vaccine doses upon exit of the study, the woman will be offered all extra doses to make sure she is fully vaccinated. By the end of The KEN SHE Study, all enrolled women will be fully vaccinated for both HPV and meningitis.