How Shape Memory Alloys work,
And how the SMA’s are "trained"
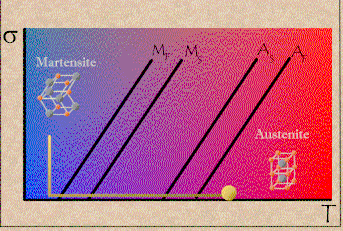
Shape memory alloys display two distinct crystal structures or phases. Temperature and internal stresses (which play a part in super-elasticity) determine the phase that the SMA will be at. Martensite exists at lower temperatures, and austenite exists at higher temperatures. (Click here to learn more about martensite and austenite). When a SMA is in martensite form at lower temperatures, the metal can easily be deformed into any shape. When the alloy is heated, it goes through transformation from martensite to austenite. In the austenite phase, the memory metal "remembers" the shape it had before it was deformed. From the stress vs. temperature graph below, one can see that at low stress and low temperature, martensite exists. At higher temperature and higher stress, austenite exists.

Stress vs. Temperature graph for NiTinol
Memory alloys also demonstrate great rates of super-elasticity. For example, eyeglass frames are in a martensite phase. Bending the arms in half (at room temperature) introduces a phase change at the bend to austenite. Austenite is not stable at room temperature, and because systems always seek lower energy states, the austenite will change back to the martensite phase, and to do this, the arm must bend back.
The most common memory metal is called NiTinol, consisting of equal parts of nickel and titanium.
The table below displays alloys having shape memory effects.
|
Alloys having a shape memory effect |
||||
|
Alloys |
Composition |
Transformation |
||
|
Atomic or Weight Percent |
Temperature Range (Celsius) |
|||
|
Ag - Cd |
44/49 at.% |
-190 to -50 |
||
|
Au - Cd |
46.5/50 at.% |
30 to 100 |
||
|
Cu - Al - Ni |
14/14.5 wt% |
-140 to 100 |
||
|
3/4.5 wt% |
||||
|
Cu - Sn |
approx. 15 at% |
-120 to 30 |
||
|
Cu - Zn |
38.5/41.5 wt% |
-180 to -10 |
||
|
Cu - Zn - (Si,Sn,Al) |
a few wt.% of (Si, Sn,Al) |
-180 to 200 |
||
|
In - Ti |
18/23 at% |
60 to 100 |
||
|
Ni - Al |
36/38 at% |
-180 to 100 |
||
|
Ni - Ti |
49/51 at% |
-50 to 110 |
||
|
Fe - Pt |
approx. 25 at% Pt |
approx. -130 |
||
|
Mn - Cu |
5/35 at% Cu |
-250 to 180 |
||
The memory transfer temperature is the temperature that the memory metal or alloy changes back to the original shape that it was before deformation. This temperature can be very precise, within 1 or 2 degrees of the desired temperature.
Training
Heating is the only way that most memory metals retain their original shape. Since heat is the property that determines the shape of the metal, heat is the first property used for manipulation for formation. If an alloy is subjected to the same heating and deformation, the alloy will begin to acquire two-way training. The treatment for a NiTinol wire is, for example:
1. The wire is hot/cold worked (stretched) by 3% when it is in the martensite phase
2. The wire is then heated to austenite finish (AF) to recover its shape
3. The wire is then cooled to martensite
Memory transfer temperatures can be altered by slight changes in composition, and by slight changes in heat treatment.