Metal properties
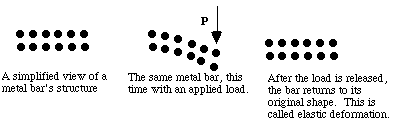
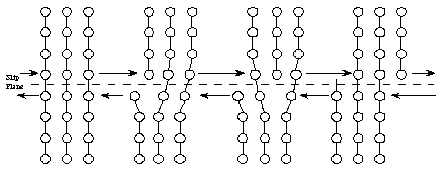
When higher stresses are applied, permanent (plastic) deformation occurs. For example, when a paper clip is bent a large amount and then released, it will remain partially bent. This plastic deformation involves the breaking of bonds, often by the motion of dislocations. See Figure 8. Dislocations move easily in metals, due to the delocalized bonding, but do not move easily in ceramics. This largely explains why metals are ductile, while ceramics are brittle.
If placed under too large of a stress, metals will mechanically fail, or fracture. This can also result over time from many small stresses. The most common reason (about 80%) for metal failure is fatigue. Through the application and release of small stresses (as many as millions of times) as the metal is used, small cracks in the metal are formed and grow slowly. Eventually the metal is permanently deformed or it breaks (fractures).