Contact Us
Email The Weinstein LaboratoryPublications (Selected)
A. McDonough & J. R. Weinstein. Role of Microglia in Ischemic Preconditioning. Glia (2019), 1-18, https://doi.org/10.1002/glia.23695A. McDonough, S. Noor, R.V. Lee, R. Dodge III, J.S. Strosnider, J. Shen, S. Davidson, T. T. Möller, G.A. Garden and J. R. Weinstein. Ischemic Preconditioning Induces Cortical Microglial Proliferation and a Transcriptomic Program of Robust Cell Cycle Activation. Glia (2019), 1-19, https://doi.org/10.1002/glia.23701A. McDonough, R.V. Lee, S. Noor, C. Lee, T. Le, M. Iorga, J.L.H. Phillips, S. Murphy, T. Möller and J.R. Weinstein. Ischemia/Reperfusion Induces Interferon Stimulated Gene Expression in Microglia. Journal of Neuroscience (2017), 37(34): 8292-8308, PMID 28747383. A. McDonough, R.V. Lee and J.R. Weinstein. Microglial Interferon Signaling and White Matter. Neurochemical Research, (2017), 42(9), 2625-38, PMID 28540600 R.G.W. Staal, J.R. Weinstein, M. Nattini, M. Cajina, G. Chandresana and T. Möller. Senicapoc: Repurposing a drug to target microglia KCa3.1 in stroke. Neurochemical Research, (2017), 42: 2639-45 A. McDonough and J.R. Weinstein. Neuroimmune Response in Ischemic Preconditioning. Neurotherapeutics, (2016), 13(4): 748-761. PMID: 27525700 M.A. Hamner, Z. Ye, R.V. Lee, J.R. Colman, T. Le, D.C. Gong, B.R. Ransom and J. R. Weinstein. Ischemic Preconditioning in White Matter: Magnitude and Mechanism. Journal of Neuroscience (2015) 25:35 (47): 15599-611. PMID: 26609155 J.R. Weinstein*, Y. Quan*, J.F. Hanson, L. Colonna, M. Iorga, S. Honda, K. Shibuya, A. Shibuya, K.B. Elkon and T. Möller. IgM-Dependent Phagocytosis in Microglia is Mediated by Complement Receptor 3, not Fc/ Receptor. Journal of Immunology (2015), 1:195(11): 5309-17. PMID: 26500348; *equal contribution K.J. Becker, R.V. Lee, J. Schulze, D. Zierath, P. Tanzi, K. Cain, A. Dressel, D. Shibata, and J.R. Weinstein. Post-Stroke Fatigue: Hints to a Biological Mechanism. Journal of Stroke and Cerebrovascular Diseases (2015), 24(3): 618. PMID: 25542762. W. Su, S. Hopkins, N.K. Nesser, B. Sopher, A. Silvestroni , S. Ammanuel, S. Jayadev, T. Möller, J.R. Weinstein and G.A. Garden. The p53 transcription factor modulates microglia behavior through microRNA-dependent regulation of c-Maf. Journal of Immunology (2014) 192(1): 358-66. PMID: 24319262. PMCID: PMC4195583. J.R. Weinstein, J. Schulze, R.V. Lee, P. Tanzi, D. Zierath, K. Cain, P. Mitchell, R. Cohen and K.J. Becker. Functional Polymorphisms in Toll-like Receptor 4 are Associated with Worse Outcome in Acute Ischemic Stroke Patients. NeuroReport (2014) 28; 25(8): 580-4. PMID: 24784586. PMCID: PMC4009512. K.J. Becker, D. Dankwa, R.V. Lee, J. Schulze, D. Zierath, P. Tanzi, K. Cain, A. Dressel, D. Shibata, and J.R. Weinstein. Stroke, IL-1ra, IL1RN, Infection and Outcome. Neurocritical Care (2013), 21(1): 140-6. PMID: 24233813. PMCID: PMC4161032. J.R. Weinstein, I.P. Koerner and T.Möller. Microglia in Cerebral Ischemia. Future Neurology, (2010), 5(2): 227-246. PMID: 20401171. PMCID: PMC2853969. Y. Quan, T. Möller and J.R. Weinstein. Regulation of Fcgamma Receptors and Immunoglobulin G-mediated Phagocytosis in Mouse Microglia. Neuroscience Letters (2009), 16:464(1):29-33. PMID: 19679164; PubMed Central PMCID: PMC2747046. C.J. Creutzfeldt*, J.R. Weinstein*, K.J. Becker, W.T. Longstreth, T.O. McPharlin and D.L.Tirschwell. Prior Antiplatelet Therapy, Platelet Infusion Therapy, and Outcome after Intracerebral Hemorrhage. Journal of Stroke and Cerebrovascular Diseases (2009), 18(3): 221-228. PMID: 19426894. PMCID: PMC2830090. *equal contribution J.R. Weinstein, M. Zhang, M. Kutlubaev, R. Lee, C. Bishop, H. Andersen, U. Hanisch and T. Möller. Thrombin-induced regulation of CD95 (Fas) expression in the N9 microglial cell line: Evidence for involvement of proteinase-activated receptor1 and extracellular signal-regulated kinase 1/2. Neurochemical Research (2009), 34:445-452. PMID: 18686031. J.R. Weinstein, Sarah Swarts, Caroline Bishop, Uwe-Karsten Hanisch and Thomas Möller. Lipopolysaccharide is a frequent and significant contaminant in microglia-activating factors. Glia (2008), 56:16-26. PMID: 17910052. PMCID: PMC2926344. J.R. Weinstein, S. Hong, J.D. Kulman, C. Bishop, J. Kuniyoshi, H. Andersen, B.R. Ransom, U. Hanisch and T. Möller. Unraveling Thrombin’s true microglia-activating potential: Markedly disparate profiles of pharmaceutical-grade and Commercial-grade thrombin preparations. Journal of Neurochemistry (2005), 95: 1177-87. PMID: 16271051. S. Balcaitis, J.R. Weinstein, S. Li, J. Chamberlain and T. Möller. Expression of proteolytically activated receptors in mouse microglial cells. Glia (2005), 50: 48-55. PMID: 15625717. J.R. Weinstein, A.L. Lau, L.F. Brass and D.D. Cunningham. Injury related factors and conditions down-regulate the thrombin receptor (PAR-1) in a human neuronal cell line. Journal of Neurochemistry (1998), 71(3): 1034-1050. PMID: 9721728. J.R. Weinstein, S.J. Gold, D.D. Cunningham and C.M. Gall. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and co-distribution with prothrombin mRNA. Journal of Neuroscience (1995), 15(4): 2906-2919. PMID: 7722637. Click Here for a Complete List of Published Work in MyBibliography (NCBI) The Weinstein LaboratoryDr. Jonathan Weinstein is the principal investigator of the Weinstein Laboratory in the Department of Neurology, University of Washington School of Medicine. The main focus of research in the Weinstein lab is to elucidate the role of microglial cell activation in the neuroinflammatory response associated with ischemic preconditioning and stroke. Ischemic preconditioning (IPC) in the brain is a robust neuroprotective phenomenon in which a brief ischemic exposure increases resistance to the injurious effects of subsequent prolonged ischemia. Microglia, the brain's resident tissue macrophages, are the primary mediators of neuroinflammation and are critical in the pathophysiology of stroke. Mechanistic information on the function of microglia in ischemia is limited and the role of microglia in IPC is unknown. All projects in our laboratory focus on characterizing the role of microglia in both IPC and stroke.
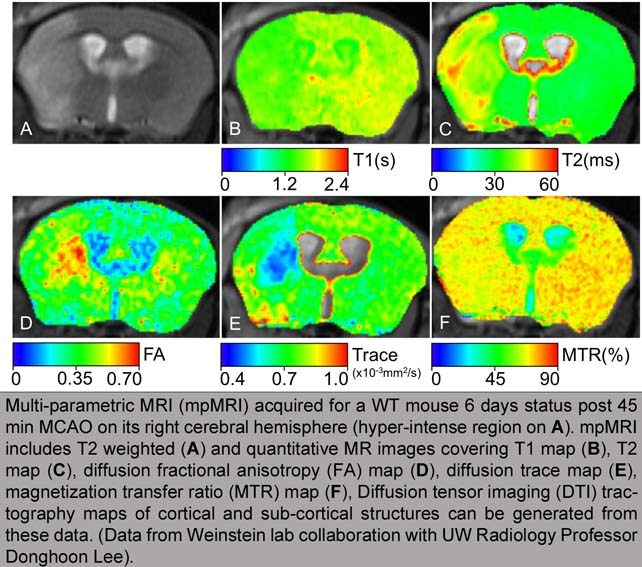
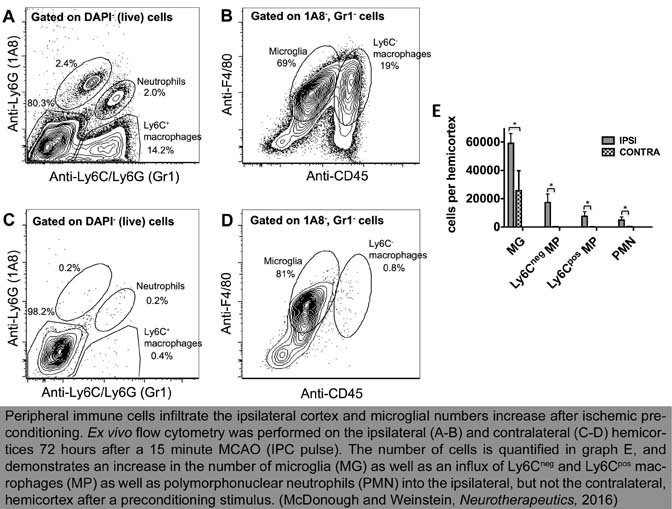
Stroke is the leading cause of serious long-term disability in the United States and few stroke patients qualify for the only FDA approved therapy, IV tPA. Thus, our ultimate goal is to improve the understanding of the mechanisms of neuroinflammation in stroke and identify possible molecular targets for therapeutic intervention. The Weinstein Lab employs both in vivo and in vitro experimental models of ischemia to study microglial responses. For our in vivo studies, we couple the mouse middle cerebral artery occlusion (MCAO) stroke model with ex vivo flow cytometric isolation of microglia from cortex. In order to determine infarct volume in our stroked mice we use both MRI and standard histological methods.
We carry out cell targeted microarray analyses on the sorted cortical microglia. Using this approach we are able to compare the microglial response to IPC/ischemia in wild-type mice with that of microglia in selected knockout and transgenic lines. Correspondingly, we are able to elucidate the effects of experimental treatments on a variety of outcome parameters. For our in vitro experimental paradigm we expose cultured primary mouse microglia to hypoxic/hypoglycemic conditions and then we monitor an array of experimental parameters. Past studies have included characterizing the cellular consequences of microglial activation by the blood coagulation proteinase thrombin -- an important factor in stroke pathophysiology. Our more recent work has implicated both Toll-like receptor-4 (TLR4) and type 1 interferon (IFN)-stimulated genes as key mediators of the microglial response to ischemia/IPC. Both TLR4 and the IFN family of cytokines are recognized as key components of the innate immune response. Ongoing projects in our laboratory are examining how disruption of the TLR4 and/or type 1 IFN signaling pathways, specifically in microglia, can affect both IPC and stroke.
Weinstein Laboratory
2017 UW Undergraduate Research Mentor Award recipient Post-doctoral FellowsAshley McDonough, Ph.D. Research ScientistsBrendan Schweitzer
Undergraduate Research AssistantsNikhil Patel Emma Lascar Haneul Ryou Lisa Young Ravneet Ranu
UW CollaboratorsBruce Ransom Lab Kyra Becker Lab Gwenn Garden Lab Suman Jayadev Lab Thomas Moeller Sean Murphy Lab
Lab AlumniChloe Lee Danielle Zierath Shahani Noor Richard Lee Michael Iorga Jamie Coleman Jessica Hadwin Anna Savos Thu Le Michelle Loprieno
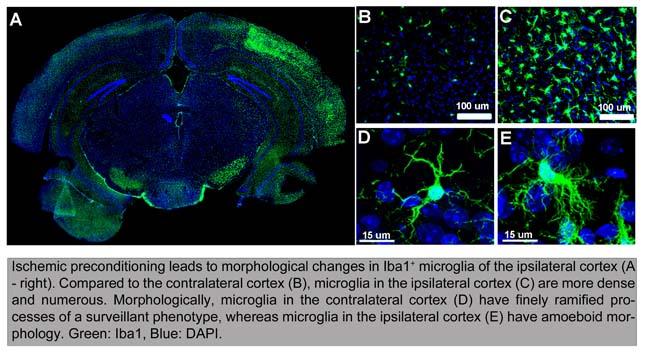
Medical Student Research Fellow AlumniDavin Gong Josiah Hanson Ryan Dodge UW Undergraduate Research Assistant AlumniRachel Arnold Graham Stoddard Vicki Wahlstrom Jasmine Shen Victoria Wahlstrom Michael Ulate Arianna Sayyadi Stephanie Mizuno Chloe Lee Dallas Kramer Alexa Erdogan Hyoeun Kim Zukhra Siddikova Ryan Dodge Erwin Odongo Dorender Dankwa Thu Le Michael Iorga Khloe Frank Katie Ho James Strosnider Cheryl Sung Sam Sussman Hunter Phillips Heather Pemberton Huy Hoang Russell Ettinger Mathew Zhang The Weinstein LaboratoryAbout UsDr. Jonathan Weinstein is the principal investigator of the Weinstein Laboratory in the Department of Neurology, University of Washington School of Medicine. The main focus of research in the Weinstein lab is to elucidate the role of microglial cell activation in the neuroinflammatory response associated with ischemic preconditioning and stroke. Ischemic preconditioning (IPC) in the brain is a robust neuroprotective phenomenon in which a brief ischemic exposure increases resistance to the injurious effects of subsequent prolonged ischemia. Microglia (Fig. 1), the brain's resident tissue macrophages, are the primary mediators of neuroinflammation and are critical in the pathophysiology of stroke. Mechanistic information on the function of microglia in ischemia is limited and the role of microglia in IPC is unknown. All projects in our laboratory focus on characterizing the role of microglia in both IPC and stroke. Stroke is the leading cause of serious long-term disability in the United States and few stroke patients qualify for the only FDA approved therapy, IV tPA. Thus, our ultimate goal is to improve the understanding of the mechanisms of neuroinflammation in stroke and identify possible molecular targets for therapeutic intervention. |




 Principal Investigator
Principal Investigator