Betzaida Garcia
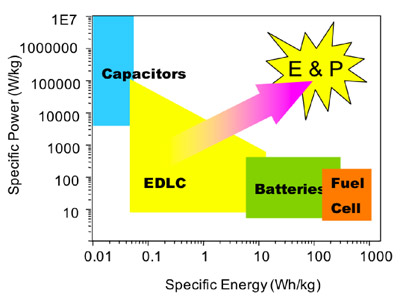
Figure 1 Ragone plot with respective energy power output from various devices. The main goal of supercapacitors is to increase both energy and power.

Figure 2 Amine catalyzed gel. Traditional aqueous recipes require many steps to be processed, gelation, aging, solvent exhange to remove the water from the gel, freeze drying or supercritical drying to preserve the nanostructures, pyrolysis and then activation. New organic recipes using amine catalyst remove some of these steps.
People - Students - Cohort 1 - Betzaida Garcia
Betzaida Garcia
Education: BS, Physics 1997 University of
Puerto Rico, San Juan
Major: Materials Science and Engineering
Advisor: Guozhong Cao
Work Experience: 11 years of experience
as an engineer for various laboratories and research oriented companies
including Fermilab, the Stanford Linear Accelerator Center (SLAC) and
FEI Company among others.
Title of Research Project: Lignin derived carbon supercapacitors
for efficient energy storage
“The major difference between a thing that might go wrong and a
thing that cannot possibly go wrong is that when a thing that cannot
possibly go wrong goes wrong it usually turns out to be impossible to
get at or repair.”
Douglas
Adams, Mostly Harmless
Since the late 1800’s the industry of petroleum, as an energy source, seemed to be infallible. It has been so reliable we ignored the inevitable fact that it is a limited resource and that some day it would come to an end. Although new alternate energy sources are emerging, petroleum has proven harder to replace than anticipated. One of the main issues is that of energy storage. Energy storage has become an increasingly important topic especially when dealing with intermittent energy sources like solar or wind power. Moreover, it is not the only issue since many applications like cars also require power delivery. One technology currently studied to meet such demands of power and energy is the electrochemical supercapacitor (ES).
Electrochemical supercapacitors are power storage devices that are used in a diverse range of consumer and industrial applications, such as advanced consumer electronics, implantable electrochemical medical devices, electric vehicles, and high performance aircraft [1]. One of the reasons that attracted considerable attention can be easily understood in terms of their specific energy and specific power as visualized in a Ragone Plot. ES fill in the gap between batteries and conventional capacitors by producing both energy and power. ES can do this since they can store large amounts of charge in their porous electrodes that after discharge is converted both into energy and power [1]. ES electrodes have very large surface areas >1000 m2/g (the area of 5 tennis courts in one gram) therefore high charge capacity compared to regular capacitors is achieved. But surface area is not all, other structural features, like pore size, are important as well.
My research focuses on new synthesis and manufacturing of electrodes for supercapacitors and batteries based on nanostructured carbon. These electrodes are produced via sol-gel (i.e. gelatin) polycondensation reactions using phenolic resins and a catalyst to tune the pore structure. Pyrolysis is then required to increase the carbon yield of the gel and make the electrodes into conductive glass like carbons. Our goal for IGERT is to expand the current sol-gel synthesis with lignin to produce nanostructured carbons based mostly on renewable sources like (furaldehyde, lignin, and amines) that are obtained from plant matter. With this new synthesis it is expected to reduce the cost of ES, promote environmentally sound energy storage (with this meaning smaller batteries can be used hence reducing toxic chemical) also using materials that are safe for the work environment (further reduce the cost of safety equipment).
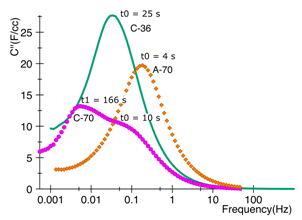
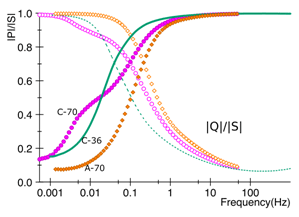
My IGERT related research involves the study of novel characterization methods using electrochemical impedance spectroscopy (EIS) to better understand the relation of the porous electrodes and the electrolyte. The electrode’s pores are in the nanometer (10-9m) range affecting the diffusion and relaxation of the electrolyte’s molecules within the pores. Current methods use only normalized values based on gravimetric and volumetric values that do not take into account the implication of geometry in the performance. EIS provide a other alternative by means of dielectric relaxation studies suitable to characterize ES’s performance [2].
The impacts of this technology are many and can include various energy source types. Currently, public transportation and the automotive industry have seen the benefits of this technology (public busses can store energy fast in each stop and electric cars can use smaller batteries and have increased power). One promising use is to store power from the electric grid especially for applications like solar and wind generated power. To achieve appropriate storage better knowledge of the microstructure is needed to increase the storage time and capacity. Hopefully this research will add to new solutions to help improve ES and replace petroleum as an energy source.
Research Plan:
• Resorcinol-furfural nanoporous carbons for electrode applications that are metal ion free are currently under way as test drive for the lignin derived gels. This work is intended to simplify the chemical methods used in previous recipes [3] and produce stronger porous electrodes that can be machined (Other glassy carbons tend to be brittle and break easily under stress).
o Simplification of current synthesis and processing methods include:
• Removal of post processing steps like the solvent exchange and oxidation at high temperatures (900 ºC) under CO2.
• Provide options to recycle the solvent removed from the gel during the drying process.
• Ultimately, to produce the lignin nanoporous carbon a better understanding of the polymerization is necessary. Lignin polycondensation would be a continuation of the previous work with amine-catalyzed gel. This study is currently under progress.
(See left for figures.)
References
B. E. Conway, Electrochemical Supercapacitors, Plenum Publishing, New
York, (1999)
B. B. Garcia, A. M. Feaver, Q. Zhang, R. D. Champion, G. Cao, T. T.
Fister, K. P. Nagle and G. T. Seidler, Journal of Applied Physics, 104,
014305 (2008).
A. Feaver and G. Z. Cao, Carbon, 44, 590 (2006).
S. Sepehri and B. Batalla, G. Cao, Uniform porosity in modified carbon
cryogels, in Nanoporous Materials Proceedings of the 5th International
Symposium, World Scientific Publishing, Singapore, Vancouver BC (2008).