Kurt Spies

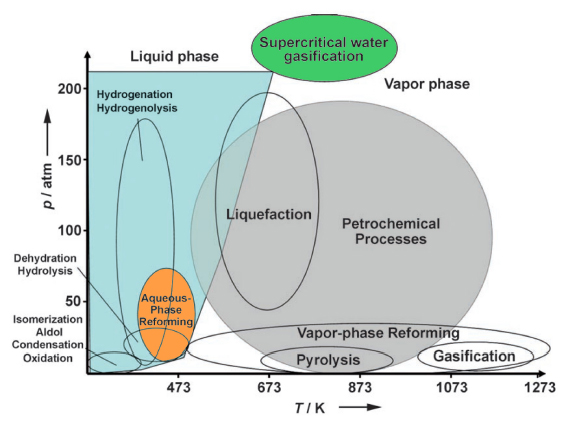
Figure 1: This figure borrowed from Chheda et al. shows different biomass processing technologies relative to petroleum processing as a function of temperature and pressure. Aqueous Phase Reforming, shown here in orange, is a relatively low temperature, low pressure process. Supercritical water gasification, shown here in green (the research focus of Ken Faires) is in a high pressure, medium temperature area of the chart.[5]
People - Students - Cohort 1 - Kurt Spies
Kurt Spies
Education: BS, Chemical Engineering 2006 Oregon State
Major: Chemical Engineering
Advisor: Eric Stuve
Title of Research Project: Aqueous-phase biomass reforming
for renewable hydrogen generation
Today’s world energy needs are met primarily by fossil fuels, nuclear energy and hydro-power [1]. Current trends of increasing population and increasing energy consumption in developing countries are putting strains on today’s energy sources [1]. To reduce this strain increasing efficiencies and new energy conversion technologies are required.
One source of energy that is not fully optimized is biomass. It is the fuel of choice for non-industrialized countries however it is mainly consumed by combustion to harness heat and in some cases electricity [1]. Combustion is inefficient, produces several pollutants and drives unsustainable harvest. Conversion of biomass into a fuel that could be used by the transportation sector would be a major technological achievement.
Aqueous Phase Reforming (APR) by definition is any reforming reaction occurring in the presence of liquid water. More specifically this reaction was designed and pioneered by Dumesic et al. at the University of Wisconsin for the generation of hydrogen, alkanes, and other chemicals from biomass derived carbohydrates [2, 3, 4]. The reaction occurs at temperatures around 500 K ( 230 C) and at sufficient pressures to have liquid water, 35-50 atm (3.55-5.07 MPa) [5]. The temperature is optimized to maximize the formation of the desired product while limiting the formation of irreversible side products. The specific physical parameters of the system are set based on the reaction of choice, feedstock, concentration, and catalyst [5].
Aqueous Phase Reforming offers improvements over traditional technology by both reducing the amount of biomass needed to generate an equivalent amount of energy and by converting it to forms that could be used as transportation fuel. The two possible products for APR are hydrogen which could be harnessed to power a fuel cell, or alkanes which could be used in a traditional combustion engine.
(See left for Figure 1.)
Research Plan: The initial plan for this project will be to design and produce an Aqueous Phase Reforming reactor for running experiments. The major design constraint for the reactor is the volume of liquid and gas products generated for measurement. The technique used to monitor the reactor will be a Gas Chromatograph( GC). The required experimental resolution will dictate the reactor flow rate.
The focus of this work will be to analyze the production of hydrogen and alkanes as a function of catalyst type and conditions. We plan to first validate the hypothesis that APR is a more efficient way to harness energy from woody biomass than combustion. From there experiments will be designed study conversion, byproduct production and catalyst lifetime. Future work on this project could look at catalytic mechanisms for different catalyst types for the aqueous phase reforming system.
Broader impacts of research: Aqueous Phase Reforming
of biomass has implications for use as a rural fuel generation system
for emergencies, or as a way to replace fossil fuels. This research
seeks to determine the viability of the technology for this purpose.
The possibility of using APR for generating hydrocarbons has implications
to replace oil in cars without having to change technology of the combustion
engine.
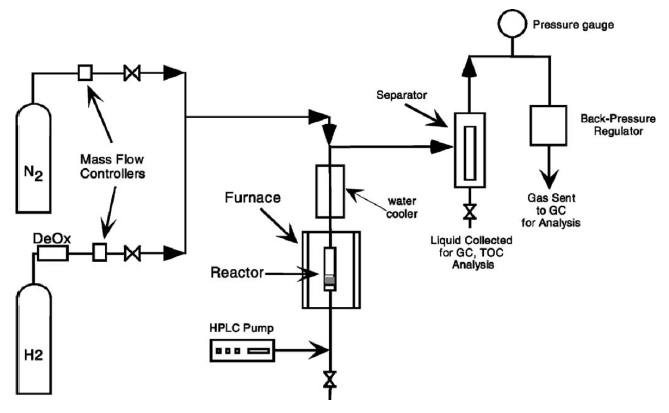
(See left for Figure 2)
References
[1] “The future of energy: The power and glory,” Economist, pp. 3–4,
June 21st 2008.
[2] R. D. Cortright, R. R. Davda, and J. A. Dumesic, “Hydrogen from
catalytic reforming of biomass-derived hydrocarbons in liquid water,”
Nature, vol. 418, no. 6901, p. 964, 2002. Article 00280836 Accession
Number: 7389470; Cortright, R.D. Davda, R.R. Dumesic, J.A.; Source Info:
8/29/2002, Vol. 418 Issue 6901, p964; Subject Term: HYDROGEN; Subject
Term: SUGARS; Subject Term: FOSSIL fuels; Number of Pages: 4p; Illustrations:
1 chart, 1 diagram, 1 graph; Document Type: Article.
[3] R. R. Davda, J. Shabaker, G. Huber, R. Cortrigh, and J. A. Dumesic,
“Aqueous-phase refoming of ethylene glycol on silica-supported metal
catalysts,” Applied Catalysis B, vol. 43, pp. 13–26, 2003.
[4] R. R. Davda and J. A. Dumesic, “Catalytic reforming of oxygenated
hydrocarbons for hydrogen with low levels of carbon monoxide,” Angewandte
Chemie International Edition, vol. 42, no. 34, pp. 4068–4071, 2003.
10.1002/anie.200351664.
[5] J. N. Chheda, G.W. Huber, and J. A. Dumesic, “Liquid-phase catalytic
processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals,”
Angewandte Chemie, vol. 46, pp. 7146–7183, 2007.