People - Students - Cohort 1 - Valerie Lieu
Valerie Lieu
Education: BS, Chemical Engineering 2007 National
Taiwan University
Major: Chemical Engineering
Advisor: Dan Schwartz
Major research project:
High Throughput Microeddy Devices for Identification and Characterization of Bioenergy Micro-organisms
In 2004, the world consumed 15 TW of energy, only 7% was supplied by renewable resources. Bioethanol is derived from renewable biomass that harvest energy through sunlight, water and carbon source in air. It can be used as an alternative energy resource to fossil fuel. Ethanol-based transportation fuels are largely compatible with current vehicles and fuel distribution infrastructure. Choices of feedstock and processing are essential for bioethanol production to reduce life cycle emissions of green house gases (GHG). The use of waste lignocellulosic biomass offers great potential for reducing GHG emissions and serve as a source of renewable energy.
To produce lignocellulosic ethanol, cellulose and hemicelluloses are first separated from lignin during pretreatment process to hydrolyse oligomeric sugars into monomers. The major sugar compositions of lignocellulosic biomass are glucose and xylose. Despite the most commonly use microorganism in industrial fermentation, Saccharomyces cerevisiae can only utilize 6- carbon sugar including glucose, mannose and galactose; and has poor utilization of 5- carbon sugar like xylose or arabinose. Agricultural and hardwood residues contain 30-35% pentose, it is essential to utilize both hexose and pentose in biomass-to-ethanol conversion.
Naturally occurring and genetically engineered xylose-utilizing microorganisms
have been investigated since the 1980s. Transgenic strains can simultaneously
ferment both glucose and xylose. However, these strains are less tolerant
to natural environment; problems including sensitivity to inhibitors
in original plant material and pretreatment process, poor utilization
of different complex, higher cost of specific media and patent protections.
The diversity of naturally occurring microorganism population has barely
been explored. Existing bulk biochemical methods are too slow to evaluate
thousands of stains in a timely manner. High throughput tools are needed
to investigate greater microbial diversity.
Research plan:
My research efforts focus on the application and design of microeddy
devices on single-cell characterization bioenergy research. There is
a continuing need for single-cell suspension trapping methods that are
easy to implement, insensitive to cell and medium properties, and can
be arrayed into highly parallel systems. We propose to develop analytical
tools that can be used to sort out and characterize single cells using
a highly parallel microeddy device.
In perior results, we showed that a microfluidic design can trap single
cells in microeddies that also accumulate reaction products. We had
developed new steady streaming flow microdevices that generate microeddies
capable of trapping and dosing reagents on cells. Trapping and dosing
suspension cells is an essential part of single cell diagnostics
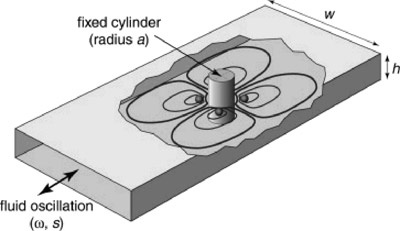
The hydrodynamic traps are created by oscillating a viscous fluid around
a cylinder in a microchannel. Steady streaming eddies are created near
the small cylinder and by audible frequency oscillations of the fluid.
This flow collects objects from the channel and traps them at specific
locations in three dimensions, as illustrated schematically in FIG X.
Strength and size of fluid recirculation (eddies) are governed by the
geometry and oscillating conditions. The oscillation creates a secondary
flow that draws particles to the trapping location near each eddy center
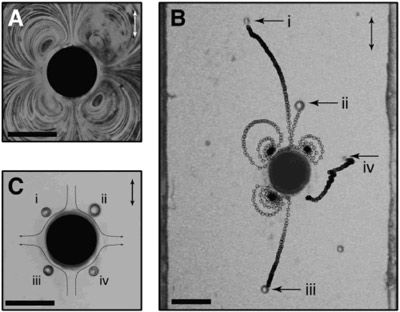
at the mid-plane of fluid channel. FIG X shows the trapping paths for
four different 22 μm polystyrene spheres. Trapping can work for dense
or buoyant particles with assorted shapes, but it is size dependant.
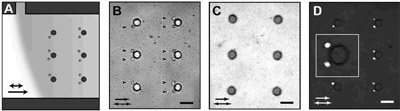
We understand much about the trapping physics and have trapped bubbles,
human T-cells, macrophage and motile phytoplankton. FIG X. shows that
many cells can be loaded simultaneously into the upstream eddies of
an array and studied using fluorescence microscopy. The major challenge
we face is how to design and implement a sorting flow field that can
collect specific microorganism from the array, and also the quantification
of different reagents in the microchannel.