Whatís so great about fuel
cells?
Isnít hydrogen a dangerous
fuel?
Can any fuel be used in a reformer?
What is the future of fuel cells?
Where can I find out more about fuel cells?
How does a fuel cell work?
There are many different kinds of fuel cells but the type we are developing is called a proton exchange membrane (PEM). Hydrogen gas (H2) reacts with a platinum catalyst were it splits into to hydrogen atoms. The hydrogen atoms are ionized (stripped of their electrons) to form protons and electrons. The protons pass through a membrane and join with oxygen on the other side. The electrons cannot pass through the membrane and so must travel through a circuit (producing DC current) to rejoin the protons and the oxygen forming water molecules.

2001 Stack Design Group
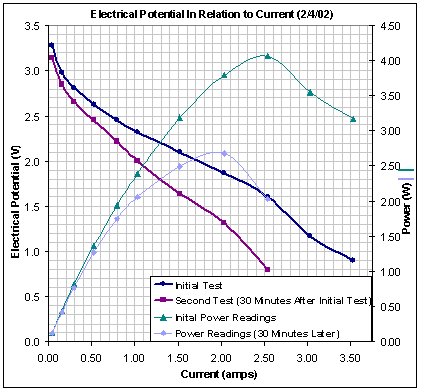
2002 Polarization Curve 5 cell stack
What's so great about fuel cells?
∑ Fuel cells are quiet and reliable with no moving
parts.
∑ They produce no emissions (other than water) when
using pure hydrogen and very light emissions when using a hydrocarbon or
alcohol fuel.
∑ They are extremely efficient compared to
conventional means of generating electricity typically 40‑50%.
∑
They are compact and lightweight making them ideal for transportation uses.
∑ PEM fuel cells have a very low operating temperature
(<100ēC) making them well suited for common applications including use for
home power.
Isn't hydrogen a dangerous fuel?
Hydrogen is a very reactive gas and can even be
explosive if not handled correctly. However this is generally true of many
industrial gasses in wide use today most notably natural gas. Gasoline is also
a very explosive and potentially dangerous fuel source if not properly handled.
Proper handling and design of equipment is an essential part of any system
using volatile fuels. In some ways hydrogen may even be safer than other gases.
Hydrogen is a very small molecule and much lighter than air. Therefore it
usually will rise and escape very quickly rather that build up in a room and
become a hazard. This is not the case with gasoline fumes, which are much
heavier than air and tend to sit low where many ignition hazards tend to be
(pilot lights, electrical outlets etc.).
Much of the public's fear about the use of hydrogen gas
has stemmed images of the much publicized Hindenburg incident. Recent evidence
from a thorough investigation suggests that hydrogen may not have played a
major role in the fire that destroyed the airship. The investigation revealed
that the gas bags holding the hydrogen were composed of cellulose nitrate and
coated with aluminum chips. Cellulose nitrate and metal chips are also the
ingredients of rocket fuel. The investigation revealed that the airship was
most likely ignited by lightning. Certainly once the fire started the hydrogen

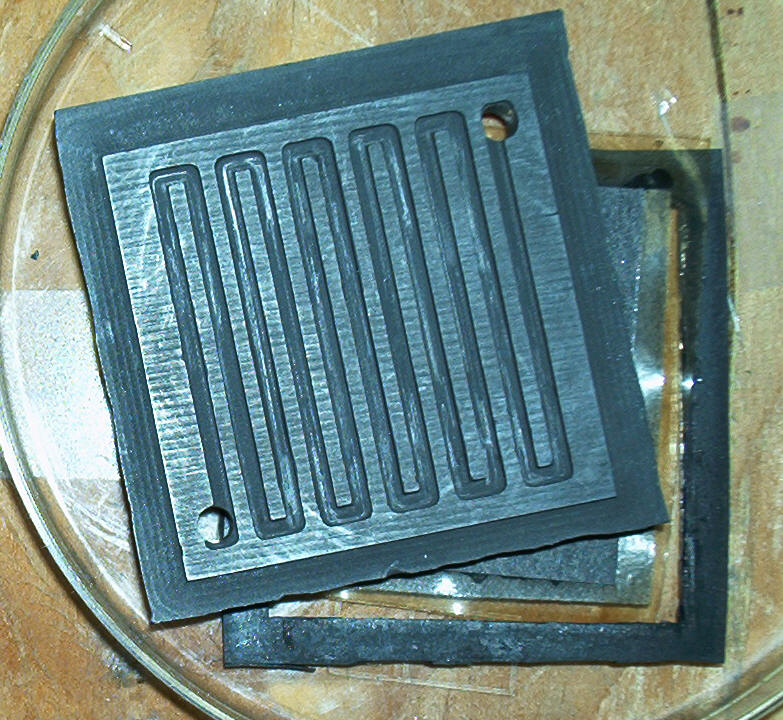
Test Stand (fuel cell is silver colored rectangle at left center)

Another view of our test stand
Single Cell used for testing (machined graphite)
Aren't fuel cells expensive?
Fuel cells have been in limited use since the Apollo
program where they were developed for use in the space program to produce water
and electricity for astronauts. They have slowly matured as a technology and
have seen increasingly diverse applications. They are still an emerging
technology but are presently on the verge of wide commercialization from many
different companies. Prices have been and will continue to fall rapidly in the
next few years as production volumes increase. The series 900 fuel cell
produced by Ballard Corp. of Vancouver Canada (one of the leading developers of
automotive fuel cells) could be manufactured including the drive motor for
$60/kw or about $4500 for a 100 horsepower drivetrain.
When discussing cost it is also important to consider the total cost of producing power. Power production today has left a legacy of pollution including acid raid, global warming, oil spills, smog etc. which are not figured into the out of pocket cost of the end users. As a clean source of energy fuel cells can be an important step to mitigating many of the problems associated with conventional production methods.
Can I buy a fuel cell?
Fuel cells are commercially available for very large (200kw) scale power production. Fuel cells the size of a washing machine which produce enough electrical power (3kw) run a typical non‑electrically heated American home, and enough waste heat to provide hot water are being beta‑tested in the northeast and in the northwest. At least one company is expecting to have a commercially available home fuel cell by 2001.
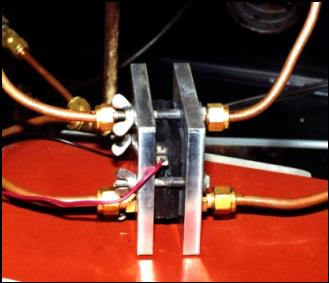
Single cell in test (wire seen is a thermocouple)

Single Cell Test and MEA preparation area.
MEA = Membrane Electrode Assembly
What is a reformer?
When fuels other than hydrogen gas are used for a fuel cell they must be processed into hydrogen gas. Small amounts of impurities such as carbon monoxide can irreversibly damage the membrane in a fuel cell. The purification process is driven by a pressure gradient (difference in pressure) with steam at high pressures typically between 50 and 250 psi". The heat input used for making the steam is derived from the combustion of a fuel gas stream. This process is responsible for a small amount of waste gases such as carbon monoxide and carbon dioxide. The carbon monoxide (a harmful pollutant) is removed in a catalytic reaction where it is converted into carbon dioxide.
Can any fuel be used in a reformer?
†††††††††††
††††††††††† In principle is possible to reform almost any hydrocarbon or alcohol into hydrogen gas. The most common processes today use methanol or natural gas, which can be cleanly, reformed to hydrogen in high yields. Fuel reformers are available for almost any common fuel (gasoline, diesel. K1 kerosene, biodiesel, propane, etc.). Fuel reforming greatly increases the flexibility of the fuel cell for uses from transportation to portable power generation. Even with a reformer a fuel cell is much more efficient and clean than a conventional heat engine.
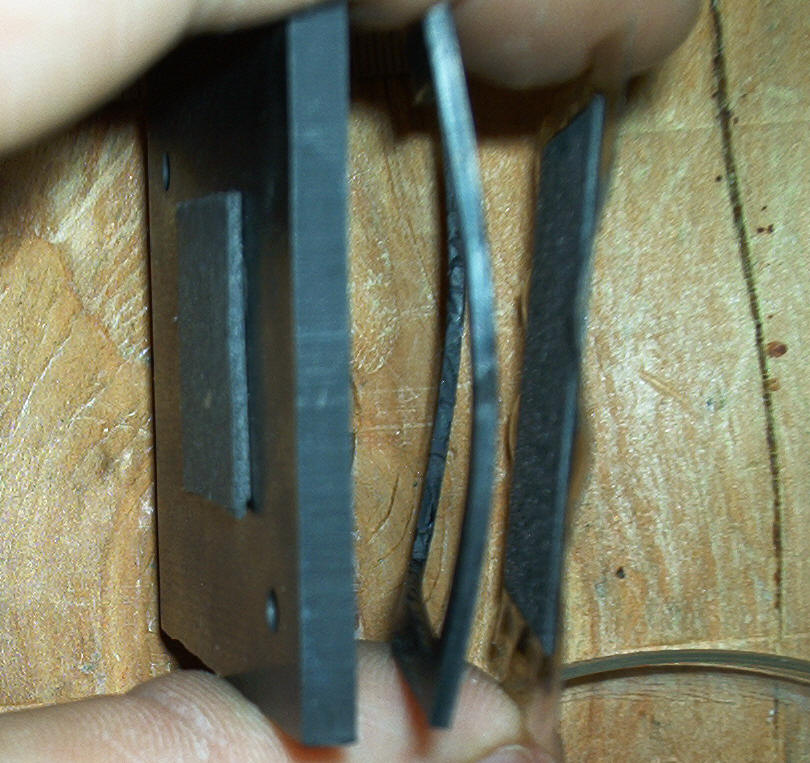
Components of single cell (left to right bipolar plate, gasket, membrane)

2001 Stack (7 cells designed and built solely by undergraduates)
What is the future of fuel cells?
Research into fuel cells is being carried out at a furious pace today. Fuel cells will certainly be used in cars and buses along with an electric drive motor. All major car companies are researching fuel cells. Fuel cells will likely replace high efficiency batteries in many small-scale applications such as cellular phones, laptop computers, camcorders, etc. in the not too distant future. Fuel cells may find their earliest widespread use right in the home. Several companies are testing fuel cells in the home at the present time and will be marketing them within a year or two. These companies are working with major utilities to train their personnel in installation and maintenance of fuel cells for the home. The use for fuel cell in the future will be virtually unlimited. The writing is on the wall and fuel cells are here to stay.
Where can I find out more about fuel cells?
Probably the best place to look for information about
fuel cells is on the World Wide Web. Our project also has a
web site that contains many
links to manufacturers and miscellaneous fuel cell projects. Try
Links for a
list of WWW Links. If you are a UW student try
UW
Resources for a list of UW books, magazine articles and microfilm that can
be found in UWís libraries. Or take the
fuel cell
engineering class offered at the UW.
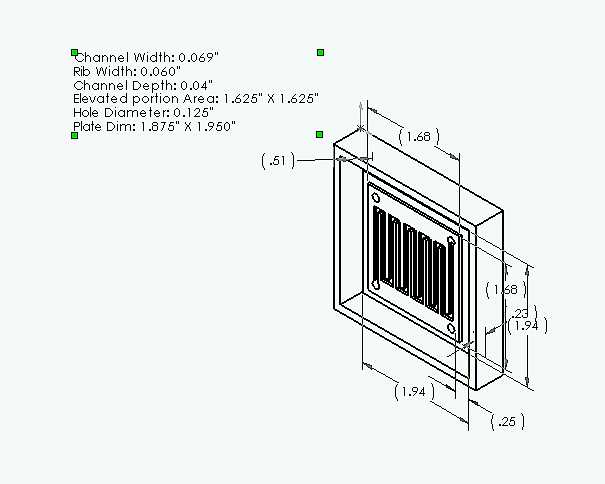
Design for single cell bipolar plate and form. See current research on conductive epoxy resin bipolar plates.