Noncoding RNAs and molecular basis of mammalian nuclear architecture
Mammalian genomes are astonishingly intricate machines that require finely tuned regulation at multiple levels. Much of this regulation is achieved through the formation of three-dimensional structures, ranging in complexity from simple DNA loops, to discrete organelle-like bodies, to the global organization of chromatin within the nucleus. Since these architectural regulatory programs collectively influence all aspects of genome function, they must be accurately reconstructed as cells divide, and precisely modulated as they differentiate. Unsurprisingly, such processess are also misrelated throughout a host of human pathologies, including neurodegenerative disorders, aging, and cancer.
Although the importance of nuclear architecture is well established, the mechanisms by which subnuclear structure regulate genome function remain opaque. Even less is known regarding the pathways by which these domains are assembled. Intriguing, noncoding RNAs (ncRNAs) have recently emerged as potentially key components in each of these processes, purportedly nucleating or modulating nuclear structures that span all levels of organization. However, deciphering the function of these putative regulatory ncRNAs has proven extremely challenging, requiring new technologies that probe and manipulate ncRNA activity in situ. To overcome these limitations, Dr. Shechner has developed a suite of such technologies, termed CRISPR-Display, CLING, and IISAAC.
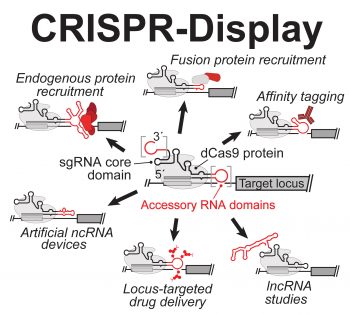
CRISPR-Display: is a versatile method for ectopically localizing ncRNA domains or ribonucleoprotein (RNP) complexes to any genomic locus, where their functions can be scrutinized or utilized (Fig. 1A). CRISPR-Display can reconstitute both natural regulatory ncRNAs and artificial ncRNA devices, and can simultaneously deploy multiple different functions to discrete genomic targets. This enables an extremely broad range of bioengineering, systems-biological, and chemical-biological applications.
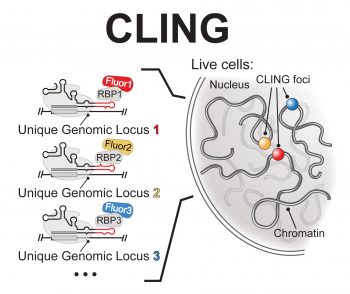
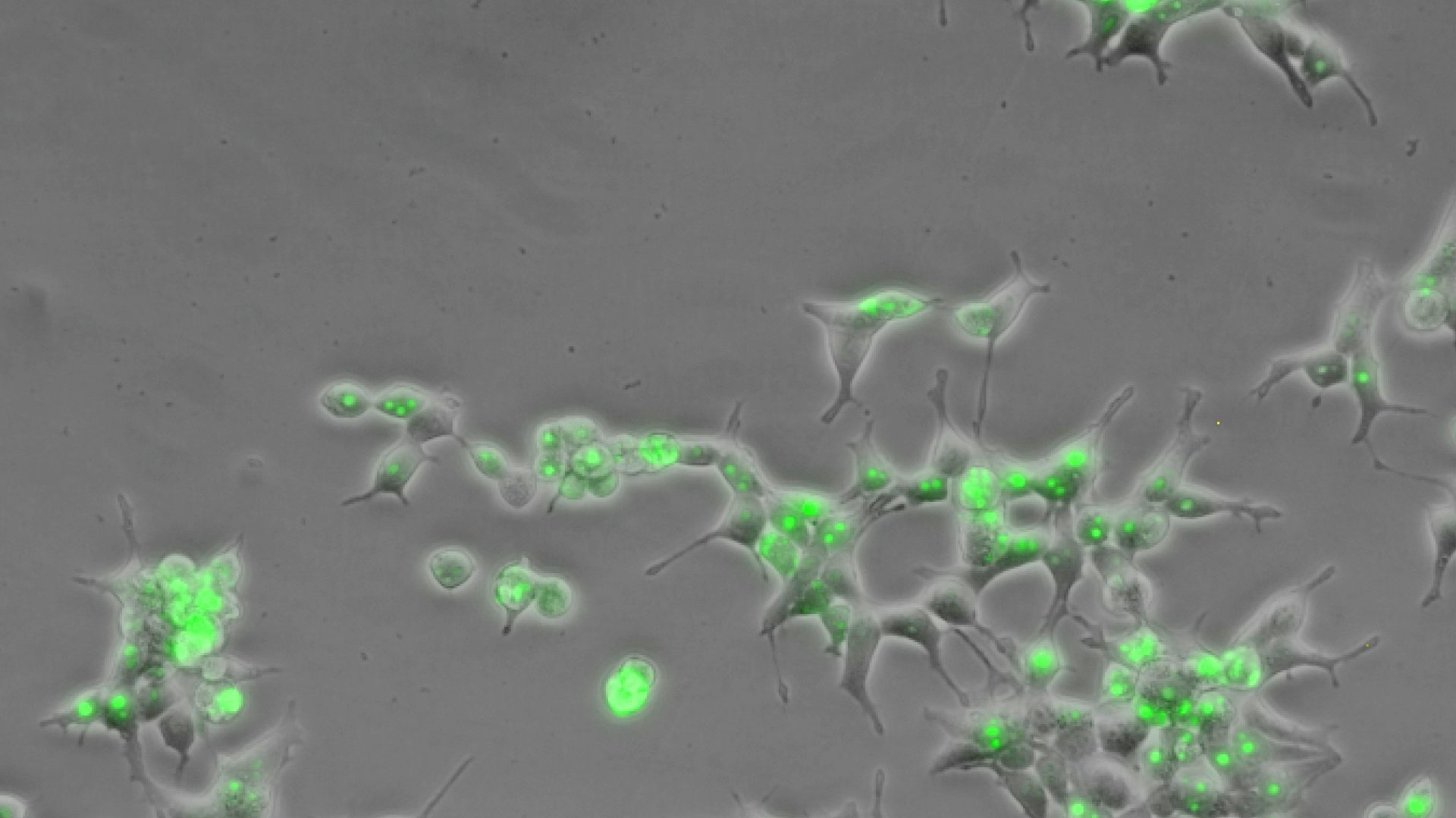
CLING (CRISPR-Display Live-Cell ImagiNG) is a method for visualizing chromatin dynamic in vivo (Fig. 1B). CLING exploits the multiplex ability of CRISPR-Display to label endogenous genomic sites with unique fluorophores, allowing the behavior of these loci-their localization, mobility, interactions with other genomic sites and subnuclear structures, etc. -to be observed in real time.
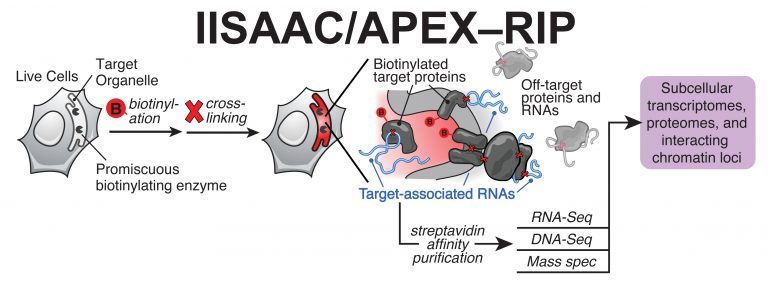
IISAAC (Interactions by In Situ Affinity-tagging And Crosslinking, also called APEX-RIP) is a universal method for mapping the sub cellular localization of RNA, transcriptome-wide Fig. 2. IISAAC uses in situ proximity-restricted biotinylation to isolate RNAs that reside in or near a target subceullular compartment, including structures that are recalcitrant to conventional biochemical fractionation. This approach can be applied in parallel to proteomic, transcriptomic and genomic analyses, allowing us to comprehensively catalog the macromolecular composition (proteins, RNAs, DNA loci) of target sub cellular regions at unprecedented breadth.
Using this toolkit-augmented with an array of biochemical, genomic, and structural biological approaches-my lab aims to decipher the molecular basis of nuclear architecture and functional scope the mammalian noncoding transcriptome. Specifically, we aim to address the following questions:
Spatiotemporal regulation in the assembly of nuclear architecture
Mammalian nuclei contain a host of long-observed, though poorly understood structures, including the nucleolus, lamina, and Cajal bodies. Many of these structures are known to vary morphologically between tissues, across diseased states, and in response to pharmacological treatment. However, since these structures often cannot be biochemically isolated, many of their fundamental attributes-including their functional scope, their biogenesis pathways, and their plasticity-remain elusive. We aim to overcome this limitation by using IISAAC to extensively catalog the composition (RNA, protein, genomic loci) of these classical subnuclear bodies, in cell culture models. These data will provide a rich foundation for “bottom-up” systems analysis, using a broad range of approaches (including genome editing and CRISPR-Display) to exhaustively dissect the contribution of each component in nuclear body assembly. Future expansions of this work will examine how the paradigms we discover are varied throughout differentiation, and in diseases such as cancer.
Structure and function of the noncoding transcriptome
Although noncoding RNAs are widely thought to regulate chromatin structure and function-including the assembly of nuclear domains-a mechanistic understanding of how they accomplish these tasks has remained elusive . We aim to address this problem by using CRISPR-Display as an activity-based screening platform to identify functional ncRNA domains, and exhaustively characterizing these novel domains through an array of biochemical, genomic, and chemical-biological approaches. This comprehensive structural and functional dissection will establish a general paradigm through which other mammalian ncRNA domains can be identified and characterized, and may reveal new routes towards novel diagnostics and therapeutic targets.
RNAs and RBPs as novel drugs and drug targets
The human proteome contains approximately 1500 RNA-binding proteins (RBPs), of which most remain uncharacterized. Misregulation of these proteins-and of their ribonucleoprotein complexes (RNPs)-has been implicated in a wide range of human pathologies, including developmental disorders, neurodegenerative diseases, and cancer. However, very few RBPs have been explored as drug targets, implying that disease-associatedRNA-protein interactions may represent a vast, untapped resource of potential therapeutic leads. To address this possibility, we are devising ways to expand CRISPR-Display as a drug-screening platform, identifying lead compounds that selectively disrupt aberrant RNA-protein interactions, and probing the mechanism of these molecules within cells.