Welcome to Our Laboratory
| Home |
| Contact Us |
| Map |
| Office of the Provost |
| UW Home Page |
![]()
| Our Research Interests |
|
Estrogens are pleiotropic hormones that act beyond the scope of their reproductive functions, and exert multiple actions on various systems that are traditionally considered non-reproductive. During the past century, the lifespan of women has lengthened to more than 80 years, but the timing of the menopause has remained fixed at about 51 years of age. Thus, women may now live about one-third of their lives in a chronic hypoestrogenic, postmenopausal state. The impact of prolonged hypoestrogenicity is a health concern because women appear to suffer from an increased vulnerability to a variety of diseases under hypoestrogenic state. Over the past two decades numerous basic science, observational and retrospective studies have supported the concept that estrogen therapy (ET) of postmenopausal women protects against age-related diseases including cardiovascular disease and neurodegenerative conditions associated with stroke. Consistently, the absence of ovarian steroid hormones after the menopause makes postmenopausal women more vulnerable to both cardio- and cerebrovascular diseases compared to age-matched cycling women. However, recent results from the Women Estrogen Stroke Trial (WEST) and the Women’s Health initiative (WHI) reported that ET afforded no benefit, or increased the risk for stroke. These discrepancies may have resulted from differences in (1) the dose of hormone, (2) the types of hormone used, (3) the route of hormone administration, or (4) the timing of hormone administration relative to the perimenopausal transition. Interestingly, the majority of subjects in both the WEST and WHI were reported to be postmenopausal for many years prior to the initiation of ET. In striking contrast, observational studies that reported cardiovascular benefits of hormone treatment examined women averaging 51 years of age, many of whom initiated hormone treatment in their perimenopausal period. Thus, our challenge is to determine whether alternate hormone regimens may afford protection against stroke and other neurodegenerative diseases, and to design therapies that favor beneficial actions with minimum risks. To this end, we must continue to perform studies in appropriate animal models and thereby gain a more complete understanding of the spectrum of estrogen's actions. In addition, we must probe the mechanisms that underlie its beneficial actions. Our studies on estrogen action against neurodegeneration have focused on whether estradiol plays a neuroprotective role in stroke injury. Investigators have developed animal models to investigate (1) the pathophysiology and potential treatments for stroke, and (2) the mechanisms by which these treatments act. Middle cerebral artery occlusion (MCAO) blocks blood flow to the cerebral vasculature and thus emulates ischemic stroke. Our laboratory utilizes a well characterized model of permanent MCAO to study the pathophysiology and therapeutic possibilities of ischemic injury. Permanent blockage of the middle cerebral artery at its base causes severe metabolic impairment in specific regions of the brain that characterize the "ischemic core." On the other hand, other brain areas undergo moderate metabolic impairment and are potentially salvageable by effective therapeutic agents, and they characterize the "ischemic penumbra". We have found that low, physiological doses of estradiol replacement are sufficient to exert dramatic protection in the ischemic brain. Estradiol achieves these actions by protecting neurons from programmed cell death (also called apoptosis) in the "ischemic penumbra”. Subsequently, we have begun to identify the repertoire of downstream genomic targets of estradiol action. To date, we have reported that estradiol modulates the expression of several players in the ischemic brain including members of the bcl-2 family that influence the extent of apoptotic cell death, products of immediate early genes, neuropeptides, trophic factors, and pro-inflammatory cytokines and chemokines. Recently, we have begun to investigate whether an additional mechanism by which estradiol limits the extent of brain injury and further enhances brain repair is by increasing the number of newborn neurons. Although estradiol exerts powerful protection in the ischemic brains, there are neurons that do not survive the impact of injury, especially those that die by necrosis in the “ischemic core”. We have reported that low, physiological levels of estradiol treatment, which exert no effect in the uninjured-state, significantly increase the number of newborn neurons following stroke injury. This proliferative actions of estradiol are confined to neuronal precursors and do not influence gliosis. Therefore, our findings suggest that estradiol facilitates the ischemic brain to remodel and repair after injury, by inducing generations of new neurons with the capability to replace damaged neurons.
In summary, many clinical and basic science studies have led to the understanding that estradiol acts far beyond the reproductive axis and exerts profound protective actions in the adult and aging brain. We have also begun to appreciate that not all doses or estrogen preparations act in the same manner. Thus, additional studies must be performed to determine (1) whether particular hormone preparations are more efficacious than others, (2) whether lower doses must be used to decrease the risk of cancers and other untoward effects, (3) whether older females that have been postmenopausal and hypoestrogenic for a prolonged period prior to the initiation of treatment may not benefit from HT, and (4) whether health status (e.g., prior history of diabetes, obesity, mini-infarcts) may negate the possible benefits of HT.
|
|
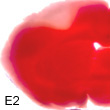
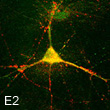
E2 powerfully protects the ischemic brain.
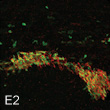
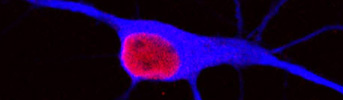
E2 enhances neurogenesis after stroke injury.
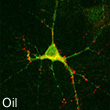
E2 induces synaptic structural changes in the hippocampus.
|
||