I.5: Homotropic Allosterism
Here, we use the random-ordered binding model to describe how common forms of atypical (non-hyperbolic) allosteric behavior may arise. We focus here on homotropic allosterism, where multiple molecules of a singly chemical species can bind the enzyme.
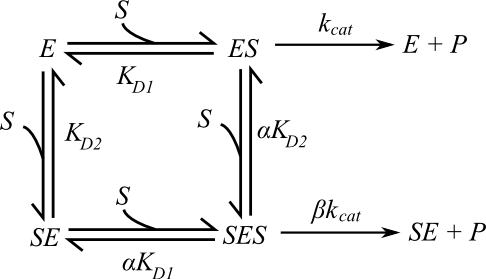
We know that the scheme above, showing a two-site random-ordered model, is described by the following velocity equation:

Different combinations of the parameters α and β can produce hyperbolic or allosteric behavior. For instance, if we set KD1 = KD2 = 5 μM with α = 1 (no cooperativity in binding) and β = 1 (ES and SES have identical activity), the overall velocity equation corresponds to a hyperbola with an apparent KM of 5 μM.
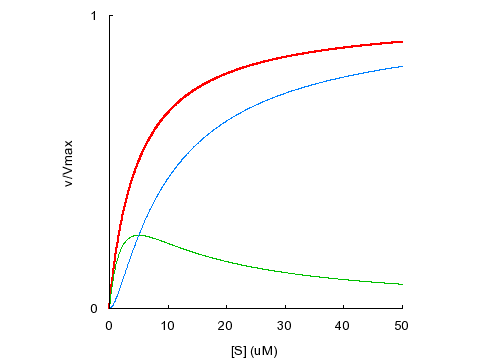
KD1
= KD2 = 5 μM, α = 1, β = 1
Total
velocity is shown in red, with contributions from ES in green and SES
in blue. In the absence of binding cooperativity and substrate activation, with
equal KDs for both binding sites, the velocity curve appears
hyperbolic.
Sigmoidal kinetics can be caused by positive binding cooperativity (α < 1) or by substrate activation (β > 1).
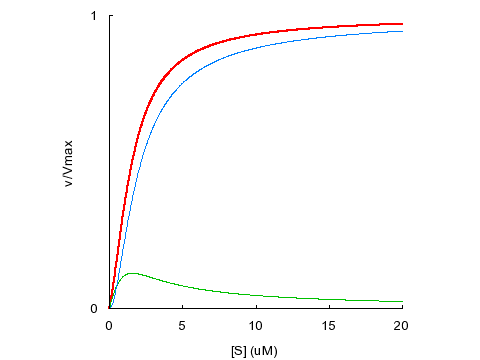
KD1
= KD2 = 5 μM, α = 0.1, β = 1
Sigmoidal kinetics can result from positive binding cooperativity.
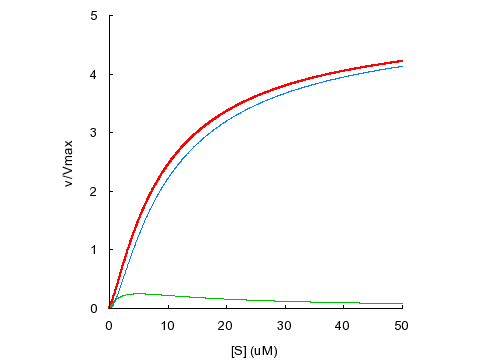
KD1
= KD2 = 5 μM, α = 1, β = 5
Sigmoidal
kinetics can also result from substrate activation, even without binding
cooperativity.
Biphasic kinetics can result from negative binding cooperativity (α > 1):
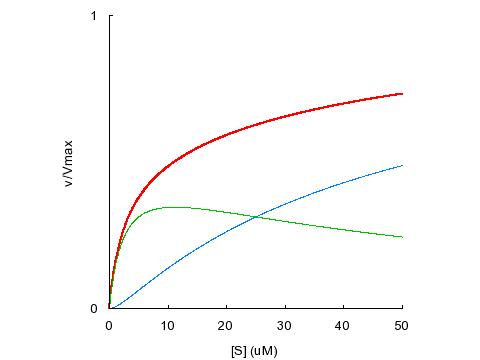
KD1
= KD2 = 5 μM, α = 10, β = 1
Substrate inhibition occurs when SES is less active than ES (β < 1):
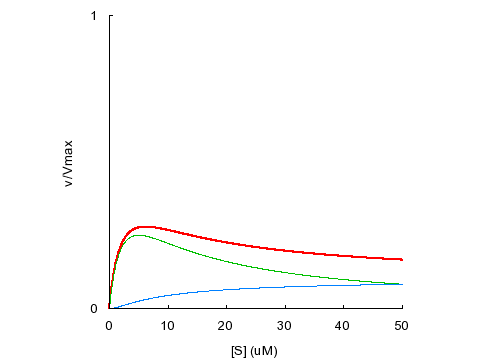
KD1
= KD2 = 5 μM, α = 1, β = 0.1