I.6 Inhibition and Activation
Random-ordered models can easily be adapted to describe many common modes of enzyme inhibition and activation by chemical species different from the substrate. The following scheme is a generalized model of inhibition that can describe competitive, uncompetitive, mixed and non-competitive inhibition, as well as heterotropic activation.

The specific terms for ES, EI and ESI are [S]/KM, [I]/KI, and [S][I]/αKMKI respectively. This leads to the following velocity equation for the general case:

This equation represents a 3-D surface with [S] and [I] as independent variables.
The parameter β describes the extent of inhibition (when β < 1) or the extent of activation (when β > 1). This system approaches competitive inhibition (where inhibitor binds to the free enzyme but not the ES complex) when α >> 1 and so KI << αKI. Uncompetitive inhibition, where I binds ES but not E, is achieved when KI >> αKI. Noncompetitive inhibition occurs when α = 1, and I binds E and ES with equal affinities. All other combinations of parameters represent mixed inhibition.
Competitive Inhibition
KM = 5 μM,
KI = 5 μM, α = 1000, β = 0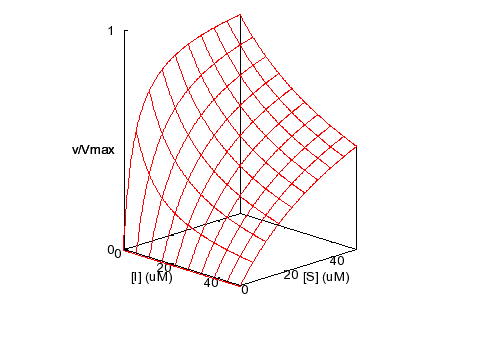
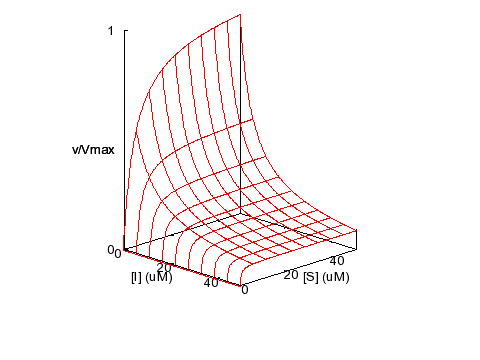
Uncompetitive Inhibition
KM = 5 μM,
KI = 5000 μM, α = 0.001, β = 0
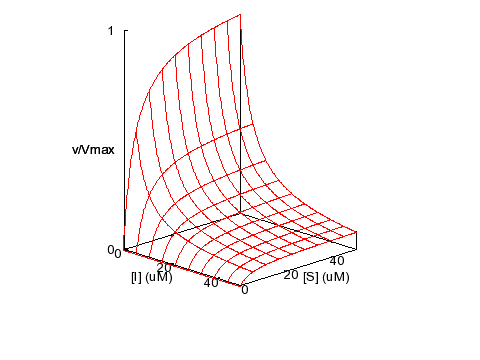
Non-competitive Inhibition
KM = 5 μM,
KI = 5 μM, α = 1, β = 0
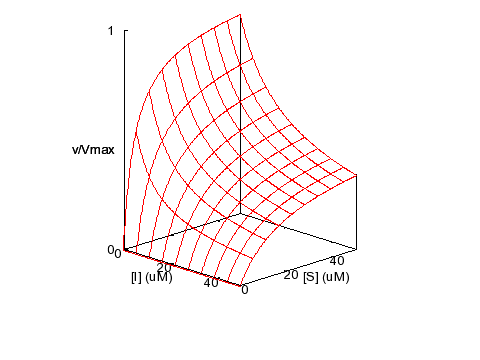
Partial Mixed Inhibition
KM = 5 μM,
KI = 5 μM, α = 5, β = 0.2
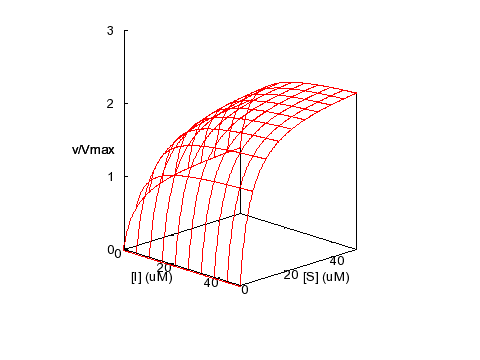
Activation
KM = 5 μM,
KI = 5 μM, α = 0.2, β = 2.5
More complex models that incorporate multiple binding sites for substrates and inhibitors can be constructed using the techniques outlined here, but this is left as an exercise for the reader.