Microstructural Inspiration from Enamel
As a bioinspired blueprint for the design of hard-yet-durable materials, teeth stand out in several ways. The outermost layer, enamel, provides a highly mineralized surface (~96% hydroxyapatite mineral, ~2% organic content, ~2% water), yet survives decades of use without any cellular repair mechanisms. The underlying dentin is softer, but serves as a durable substrate that can stop cracks in the tooth from becoming catastrophic. Understanding how these two materials function and how they are altered by diseases (e.g. age, amelogenesis imperfecta, diabetes) is one of the primary focuses of our group.
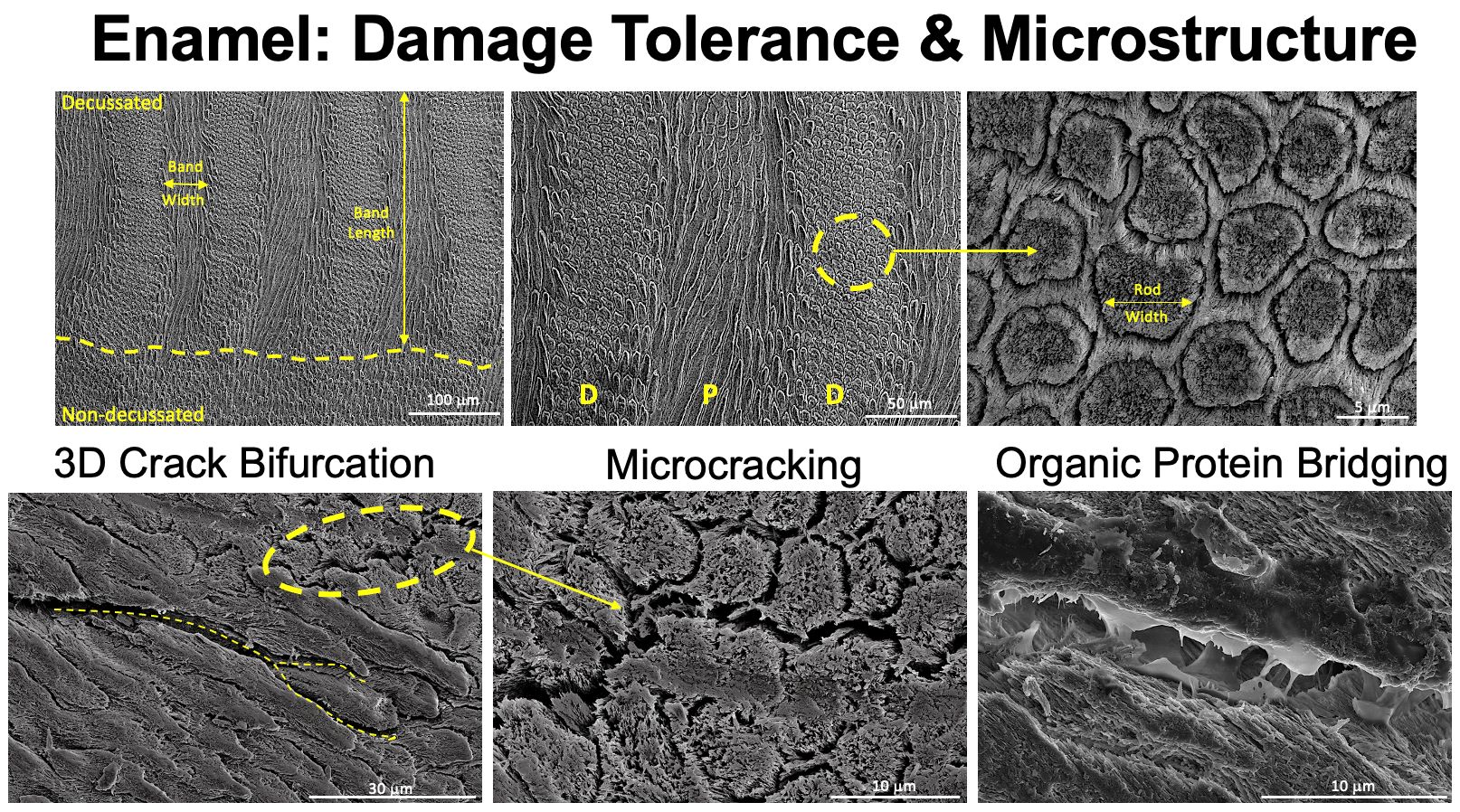
Mechanisms of crack growth resistance in enamel.
For enamel, the resistance to failure is facilitated by the complex weaving microstructure called decussation, which has been shown to be a key factor that resists the growth of incident cracks (Bajaj & Arola, 2009). This suggests that a next-generation material with high-damage tolerance could be derived from the microstructural features seen in enamel. Decussation is a feature seen across almost all of Mammalia, but characterization outside of Homo sapiens is relatively scarce in the literature. To address this, our group has collaborated with the Burke Museum of Natural History and Culture at the University of Washington to study the enamel of non-human mammals. The results suggest that the enamel tissue evolves to enable the diet of the animal, and furthermore, provides different sources of inspiration depending on the design objectives.


Synchrotron micro-computed tomogrpahy enables tracking of individual rods in three-dimensional space through the full thickness of the enamel layer. This provides accurate measurement of the variations in rod paths (i.e. pitch & yaw) for individual rods and across diazone (D+, D-) and parazone (P) bands for a variety of species.
The rod microstructures, due to their fiber-like nature, are poised to be translated into other materials through e.g., additive manufacturing. Therefore, there is great value in systematically quantifying the rod patterns from multiple species. To this end, we have used synchrotron micro- and nano-computed tomography (Marsico et. al., 2024, Guo et. al., 2024) to fully evaluate the rod structure in three dimensions. Like the mechanical properties, we observed that species with a higher bite force have a wider range of rod curvatures and more frequent oscillations in pitch variations.
Select Publications
C. Marsico, C. Renteria, J.R. Grimm, J. Fernandez-Arteaga, D. Guillen, D. Arola, A Machine Learning Approach to Quantitative Analysis of Enamel Microstructure from Scanning Electron Microscopy Images, Small Struct. (2024). https://doi.org/10.1002/sstr.202400510.
C. Marsico, J.R. Grimm, C. Renteria, D.P. Guillen, K. Tang, V. Nikitin, D.D. Arola, Characterizing the Microstructures of Mammalian Enamel by Synchrotron Phase Contrast microCT, Acta Biomater. 178 (2024). https://doi.org/10.1016/j.actbio.2024.02.038.
Z. Guo, D.P. Guillen, J.R. Grimm, C. Renteria, C. Marsico, V. Nikitin, D. Arola, High Throughput Automated Characterization of Enamel Microstructure using Synchrotron Tomography and Optical Flow Imaging, Acta Biomater. 181 (2024). https://doi.org/10.1016/j.actbio.2024.04.033.
D. Guatelli-Steinberg, C. Renteria, J.R. Grimm, I.M. Carpenter, D.D. Arola, W.S. McGraw, How mangabey molar form differs under routine vs. fallback hard-object feeding regimes, PeerJ 11 (2023). https://doi.org/10.7717/peerj.16534.
C. Renteria, J.M. Fernández-Arteaga, J. Grimm, E.A. Ossa, D. Arola, Mammalian enamel: A universal tissue and diverse source of inspiration, Acta Biomater. 136 (2021). https://doi.org/10.1016/j.actbio.2021.09.016.
M. Yahyazadehfar, J. Ivancik, H. Majd, B. An, D. Zhang, D. Arola, On the Mechanics of Fatigue and Fracture in Teeth, Appl Mech Rev 66 (2014). https://doi.org/10.1115/1.4027431.
M. Yahyazadehfar, D. Bajaj, D.D. Arola, Hidden contributions of the enamel rods on the fracture resistance of human teeth, Acta Biomater 9 (2013). https://doi.org/10.1016/j.actbio.2012.09.020.
D. Bajaj, D.D. Arola, On the R-curve behavior of human tooth enamel, Biomaterials 30 (2009). https://doi.org/10.1016/j.biomaterials.2009.04.017.
D. Bajaj, D. Arola, Role of prism decussation on fatigue crack growth and fracture of human enamel, Acta Biomater 5 (2009). https://doi.org/10.1016/j.actbio.2009.04.013.
