Permanent tissues means permanent damage
One consequence of enamel and dentin being acellular tissues is that any damage suffered during regular use or as a consequence of treatments in the dental clinic. The same is true of compositional changes, whether that be disease, caries (“cavities”), frequent consumption of highly acidic food and drink, or simply the passing of time. The dentin and enamel of samples from a senior age group are harder, stiffer, and more brittle than young adult teeth. Compositional changes are thought to be the underlying cause for this degradation in durability.
A collaboration with the Pacific Northwest National Laboratory in Richland, WA, has enabled explorations of nanoscale compositional differences in young and senior enamel using a technique called atom probe tomography. Early studies have focused on the composition of the mineral content near the outer surface of the enamel, but we are equally interested in the organic components that are believed to improve the toughness of enamel.

Spatially resolved micromechanical properties for all the three age groups from the Tooth Surface to the DEJ. (a) Apparent fracture toughness responses and (b) Brittleness distributions. Error bars are shown only for the primary group for clarity.
Chronic diseases like diabetes can also impact oral health. Using a mouse model, our group studies the influence diabetes has on the properties of dentin, providing guidance for what considerations may be necessary when treating diabetic patients.
One of the more damaging procedures that might happen at the dentist’s office is a root canal. During this procedure, diseased tissue is removed from the roots of the tooth, often at the expense of most of the dentin in the tooth. A prosthetic tooth crown is then secured into the hollowed-out root. Unfortunately, the damage to the surrounding tissue that the crown is secured is an unreliable interface, and it is not uncommon for crowns to fail after a period of ~5-7 years.
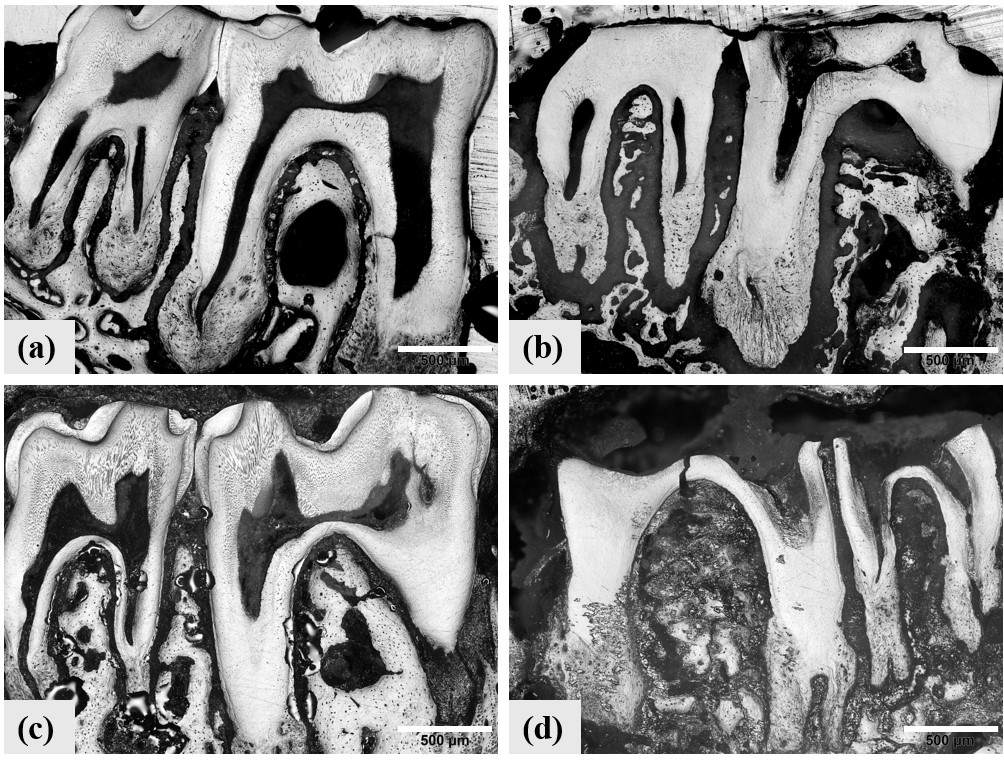
Molars from (a) group 1 nondiabetic, (b) Type 1 diabetic, (c) group 2 nondiabetic, and (d) Type 2 diabetic mice. Significant wear is evident in the crowns of diabetic teeth.
Our group is interested in thoroughly understanding how these diseases and changes affect the composition—and in turn the mechanical properties—from the micro- to nano-scale. Some of our commonly used techniques in this pursuit include Raman spectroscopy, Fourier-transformed infrared spectroscopy, nanoindentation, indentation fracture resistance, scanning electron microscopy, and atom probe tomography, among others. We have also applied machine learning algorithms (i.e. k-means clustering) to identify correlation between sub-groups that might not be apparent upon initial analysis.
Select Publications
Grimm, J., Renteria, C., Mukhopadhyay, S., Devaraj, A., Arola, D. Stratification of fluoride upatake among enamel crystals with age elucidated by atom probe tomography. Communications Materials 5 (2024). https://doi.org/10.1038/s43246-024-00709-8
C. Renteria, W. Yan, Y.L. Huang, D.D. Arola, Contributions to enamel durability with aging: An application of data science tools, J Mech Behav Biomed 129 (2022). https://doi.org/10.1016/j.jmbbm.2022.105147.
W. Yan, C. Renteria, Y. Huang, D.D. Arola, A machine learning approach to investigate the materials science of enamel aging, Dent Mater 37 (2021). https://doi.org/10.1016/j.dental.2021.09.006.
W. Yan, H. Chen, J. Fernandez-Arteaga, A. Paranjpe, H. Zhang, D. Arola, Root fractures in seniors: Consequences of acute embrittlement of dentin, Dent Mater 36 (2020). https://doi.org/10.1016/j.dental.2020.08.008.
W. Yan, C. Montoya, M. Øilo, A. Ossa, A. Paranjpe, H. Zhang, D.D. Arola, Contribution of Root Canal Treatment to the Fracture Resistance of Dentin, J Endodont 45 (2019). https://doi.org/10.1016/j.joen.2018.10.004.
C. Montoya, D. Arola, E.A. Ossa, Deformation behaviour of aged coronal dentin, Gerodontology 35 (2018). https://doi.org/10.1111/ger.12321.
W. Yan, C. Montoya, M. Øilo, A. Ossa, A. Paranjpe, H. Zhang, D. Arola, Reduction in Fracture Resistance of the Root with Aging, J Endodont 43 (2017). https://doi.org/10.1016/j.joen.2017.04.020.
M. Yahyazadehfar, D. Zhang, D. Arola, On the importance of aging to the crack growth resistance of human enamel, Acta Biomater 32 (2016). https://doi.org/10.1016/j.actbio.2015.12.038.
C. Montoya, S. Arango-Santander, A. Peláez-Vargas, D. Arola, E.A. Ossa, Effect of aging on the microstructure, hardness and chemical composition of dentin, Arch Oral Biol 60 (2015). https://doi.org/10.1016/j.archoralbio.2015.10.002.
R. Wang, S. Mao, E. Romberg, D. Arola, D. Zhang, Importance of aging to dehydration shrinkage of human dentin, Appl Math Mech 33 (2012). https://doi.org/10.1007/s10483-012-1553-8.
S. Park, D.H. Wang, D. Zhang, E. Romberg, D. Arola, Mechanical properties of human enamel as a function of age and location in the tooth, J Mater Sci Mater Medicine 19 (2008). https://doi.org/10.1007/s10856-007-3340-y.
D. Bajaj, N. Sundaram, A. Nazari, D. Arola, Age, dehydration and fatigue crack growth in dentin, Biomaterials 27 (2006). https://doi.org/10.1016/j.biomaterials.2005.11.035.
