Research Focus
1) Advancing imaging technologies:
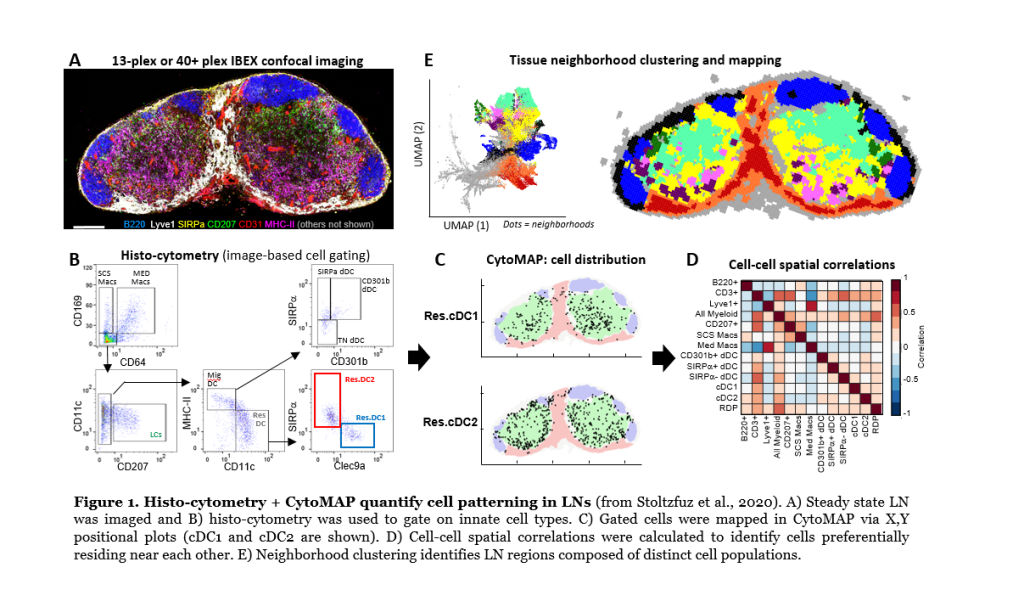
Dissociation-based tools fail to capture how cells interact and influence one another in tissues. We have developed several imaging tools to study the organization of phenotypically complex cells directly in situ. This includes image-based cellular gating with histo-cytometry, Ce3D tissue clearing and volumetric microscopy, and statistical image analysis with CytoMAP. Example of this analysis approach to detect innate cell positioning in lymph nodes is shown in Figure 1 below. Multiparameter confocal images (Figure 1A) are first used for cell population gating with histo-cytometry (Figure 1B), and this allows for positional mapping of these cells (Figure 1C; positioning of two dendritic cell types, cDC1 and cDC2, is shown). CytoMAP is next used to explore more complex features, such as cell distances to anatomical landmarks, cell-cell spatial correlations (Figure 1D), or spatial clustering of cells into regions to reveal global tissue organization (Figure 1E). We also utilize intravital 2-photon imaging to understand the dynamics of cellular motility and behavior during the steady and inflammation. Current areas of research are focused on coupling highly multiplexed imaging techniques (>40plex imaging) with quantitative analysis, as well as bridging these data with single cell RNA sequencing and Spatial Transcriptomics. Together, these techniques will help unravel the fundamental rules of immune cell homeostasis, activation, and function, as well as drive the development of next generation diagnostic tools.
2) Understanding how lymphoid tissue organization influences early immune responses to vaccination or infection:
Immune cells need to communicate with one another in order to properly respond and function. We have previously shown that lymph nodes are highly organized structures with precisely positioned innate cell populations. Current studies are using advanced imaging technologies and functional studies to understand the mechanisms of this organization, as well as how cellular positioning impacts early immune responses to vaccination, or during infection with Mycobacterium tuberculosis (collaboration with Dr. Kevin Urdahl, Seattle Children’s Research Institute). We are also investigating the role of lymphatic flow in the generation of heterogeneity in the adaptive immune response. These studies will help the development of better vaccines and host directed therapeutics.
Example of this is shown in the Figure 2 below. Data show that after immunization, lymph nodes become compartmentalized into two distinct sub-compartments, one that is rich in inflammatory monocytes and the other rich in dendritic cells (Figure 2A,B). T cells activated in monocyte-rich zones are more likely to become highly differentiated effector cells, while T cells activated in dendritic cell -rich regions are more likely to become less-differentiated early memory precursor cells (Figure 2C-E).


3) Dissecting the roles of cellular positioning in inflamed peripheral organs in the regulation of cellular function and host defense:
After initial activation in lymphoid tissues, effector T cells need to infiltrate peripheral inflamed tissues, communicate with other cells, and perform their effector functions. We are studying immune cell organization in diseased tissues, such as lung granulomas during Mycobacterium tuberculosis infection, as well as in tumors. We have found that T cells sense divergent activating vs. regulatory signals within distinct tissue microenvironments and that these signals play important roles in promoting vs. inhibiting the adaptive immune response. Current projects aim to understand mechanisms driving cellular organization in these sites, how activating vs. inhibitory microenvironments are established, and ways to manipulate these processes to promote host defense.
Example of this is shown in the figure below (Figure 3). Here, we analyzed the cellular composition and spatial organization of tumor microenvironments (TMEs) in MC38 colorectal tumors, either without treatment or with immune therapy. We find multiple different types of TMEs in tumors, some that are rich in T cells and some that have few T cells but lots of cancer cells and macrophages (Figure 3A, red vs. blue boxes). The relative composition and organization of these TMEs vary with treatment type, and this highly correlated with response to immune therapy (Figure 3B-D).

