TLC stands for “Targeted Long-acting Combination” of drugs intended to simultaneously knock out multiple drug targets within the cells of viral infected cells or occult cancer cells.
TLC products are designed to reach tissue and cell targets, taken up into the cells and persist intercellular to maximally suppress and eliminate occult cells in the body. The synchronized exposure in cells is enabled by Drug-combination Nano-Particle technology called DcNP. Injectable drug-combination product formulated in DcNP dosage form has demonstrated to provide long-acting intracellular and plasma pharmacokinetic characteristic.
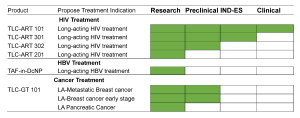
The following list contains drug combination product candidates and their research and development status toward clinical translation.
Latest publication on HIV
coming soon
coming soon
Last Update: Oct 8, 2025