New Clinical Report on Journal of Infectious Diseases
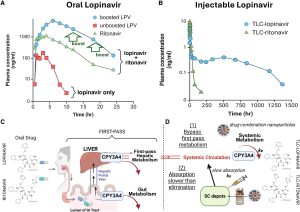
We wanted to share an exciting discovery that PK analysis of a long-acting 3-HIV drug injectable product called TLC-ART 101, containing a protease inhibitor Lopinavir (LPV) and a booster Ritonavir (RTV) (plus RT inhibitor Tenofovir) in humans suggests that booster ritonavir may not necessary. A single SC injection of LPV/RTV in TLC-ART 101 provided about 2 months of plasma LPV levels while RTV was cleared within two days. Another surprising finding is that TLC-ART 101 lasted two weeks in primate model, but 2 months in humans. Follow this link for additional details, published at Journal of Infectious Diseases.
Implication: for long acting injectable protease inhibitor products, including those of HIV and Covid19 medicine be developed without boosters such as ritonavir or cobicistat.