The TLC-ART work was highlighted in plenary talks at CROI regarding long-acting therapies. Notably, our preliminary results in nonhuman primates demonstrated persistent levels of the well-known tenofovir-lamivudine-dolutegravir (TLD) combination, now feasible as a single subcutaneous long-acting injectable. The full article is available in AIDS.
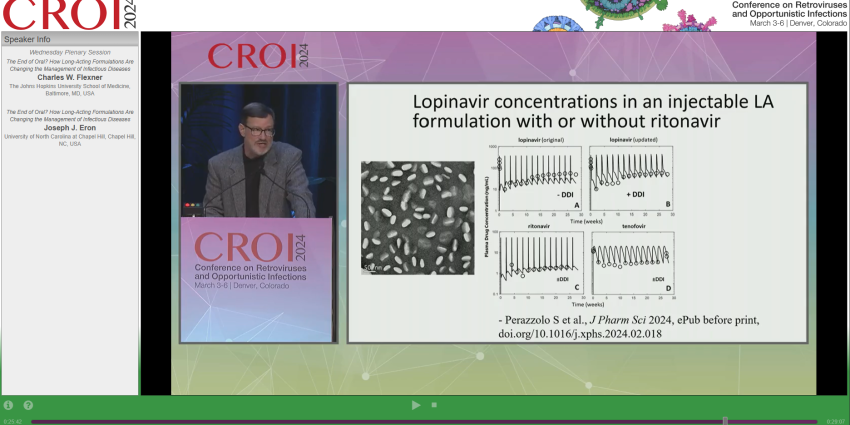
Our lab was mentioned regarding the drug-drug interaction study published on J Pharm Sci on the long-acting product 101, which includes lopinavir, ritonavir, and tenofovir. According to safety studies in nonhuman primates with six months of 101 dosing, ritonavir appears to have little influence on lopinavir plasma concentration. The hypothesis is that the subcutaneous route and lipidic coating of the particles prevent strong drug-drug interactions as seen with oral pills. So, Could this signal the end of the boosting strategy in infectious diseases?