Nocturnality in Owl Monkeys

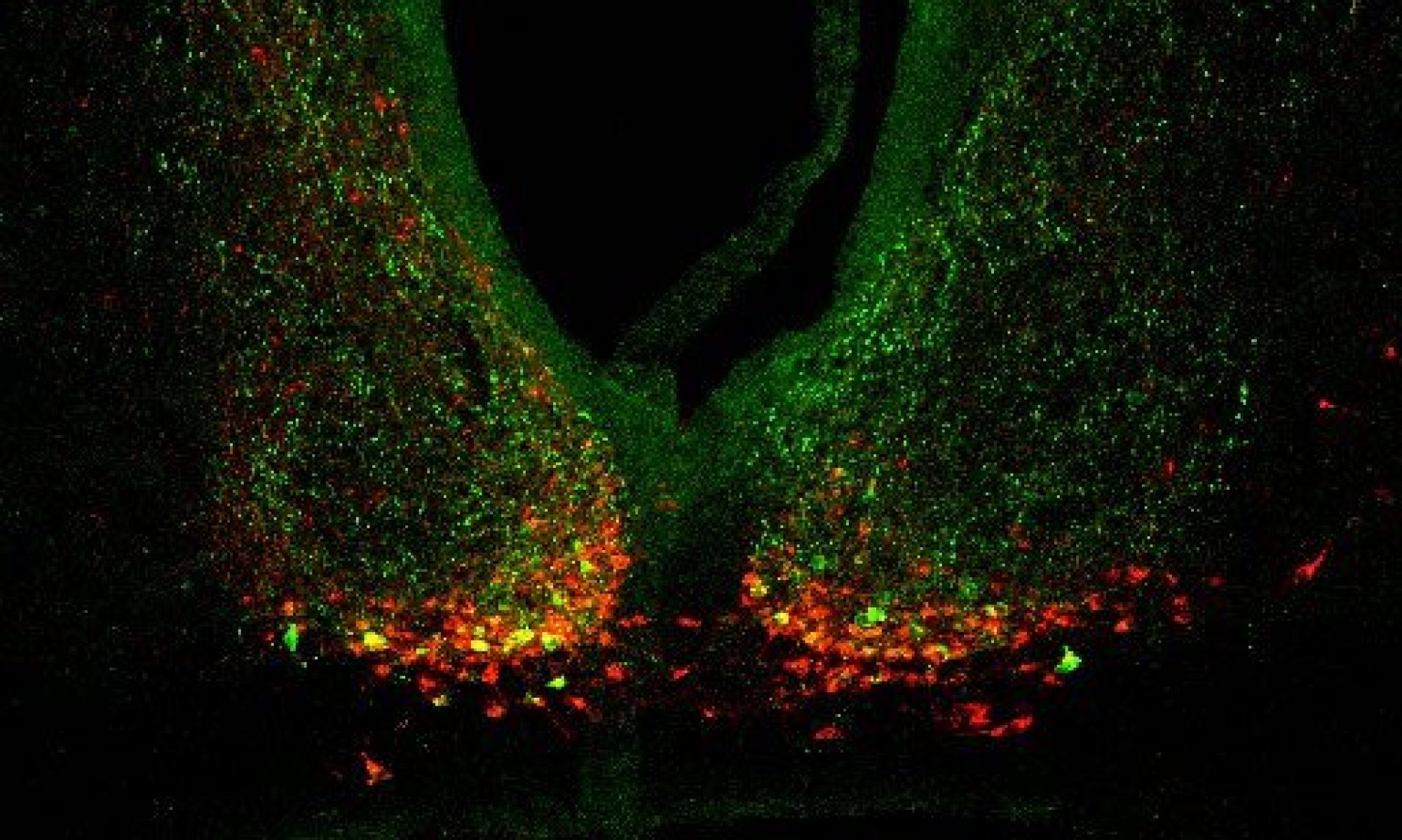
Owl monkeys (genus Aotus) represent the only taxon of monkeys that are nocturnally active. In field studies in collaboration with The Owl Monkey Project we showed that the nocturnal activity of owl monkeys in northern Argentina is dependent on the availability of moonlight. Monkeys are active during moonlit nights but they shift to a diurnal behavior with the waning moon, changing their ‘temporal niche’ predictably with moon phases. We continue field studies, as well as laboratory studies at the Keeling Center for Comparative Medicine and Research, to elucidate the physiological trade-offs of these predictable nocturnality/diurnality switches.
Locomotor activity of an Aotus azarai owl monkey in its natural environment in northern Agentina. Nocturnal activity is evident only during moonlit nights. Black circles on the left indicate new moon, WS = winter solstice, SS = summer solstice. From Fernández-Duque et al. (2010).
Searching for Human Ancestral Sleep
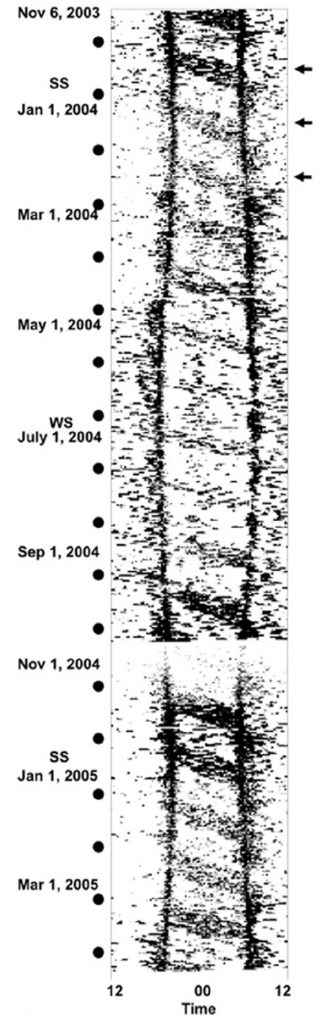
Sleep has been central to human life and its 24-h timing likely changed prominently as humans transitioned from hunting-gathering to agricultural to industrial societies that facilitated access to artificial light. We study sleep non-invasively in aboriginal communities in South America that live in pre-industrial habitats and still practice hunting gathering as part of their survival. We are specifically interested in the impact of electric light on the circadian system and the daily timing of sleep.

Sleep and Epilepsy
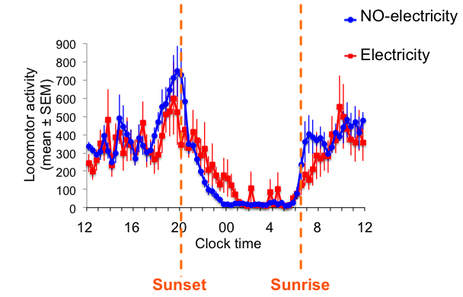
Epilepsies are among the most common neurological disorders globally. Epileptic syndromes are typically associated with sleep disorders but the neural basis for the impaired regulation of sleep is not well understood. We exploit a genetic mouse model of a severe childhood epilepsy, Dravet syndrome, to determine the mechanisms by which a genetic mutation that produces generalized seizures can also induce deficits in the circadian and homeostatic regulation of sleep. Currently, we are exploring both closed-loop electrophysiological approaches and noninvasive circadian behavioral interventions to improve sleep-related symptoms of Dravet syndrome. In collaboration with the Garden lab at UW, we are also investigating how microglia-mediated neuroinflammation drives sleep and seizure disorders in a mouse model of Alzheimer’s disease.
Scn1a+/- mice, a model of Dravet syndrome, show abnormal circdadan behavior including reduced amplitude and increased period of circadian wheel running, impaired light-induced phase shifts and slowed re-entrainment after jet lag. From Han et al. (2012).
Sleep and Academic Performance

Sleep-deprived undergraduate students.
Sleep is critical for the acquisition and consolidation of memory. One of the many important places where we rely on memory formation is in college, where students acquire, filter and consolidate large amounts of information. We non-invasively study sleep habits in high school and undergraduate students, and examine whether specific sleep patterns are associated with academic performance. Students participate of a course-based research experience in which they measure their own sleep wearing wrist activity monitors and analyze whether their sleep patterns are associated with their performance in class.
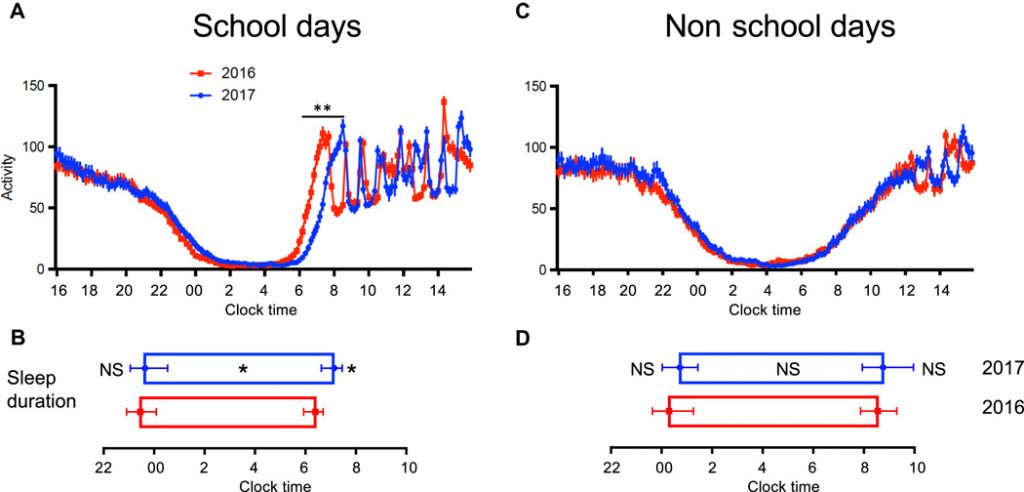
Delayed school start times in Seattle high schools result in later sleep offset and longer sleep duration (from Dunster et al. 2018)
Fear Entrainment
Circadian clocks are entrained by 24-h environmental cycles and this process assures the synchrony of circadian rhythms with the temporal environment. The light-dark cycle is the most prominent cycle entraining circadian systems but other 24-h cycles can entrain them as well. When mice or rats are housed in a cage divided between a safe nest and a foraging area, animals forage and feed during the dark phase. If the foraging area is made dangerous by applying aversive stimuli only during the dark phase, the animals switch their foraging and feeding behavior to the light phase. This diurnal behavior represents the output of an entrained circadian clock. We are currently investigating the location of this clock and the neural circuitry that underlies this entrainment.

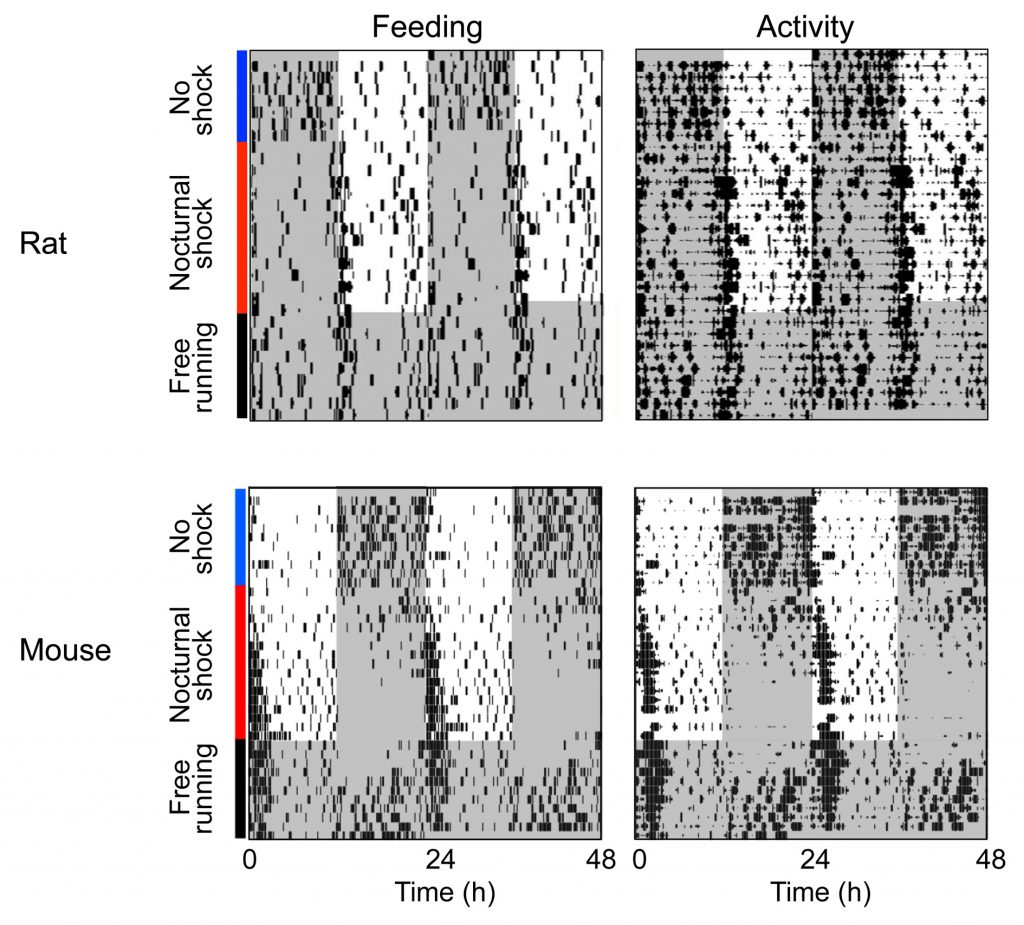
Cyclic nocturnal fear (red bar) in the foraging area induces a shift of feeding and foraging activity to the light phase. These rhythmic diurnal behaviors are the output of a fear-entrained oscillator. Rat, from Pellman et al. (2015); mouse, unpublished.
The Role of GABA in SCN Function
Nearly every neuron in the suprachiasmatic nucleus (SCN), the master circadian clock located in the mammalian hypothalamus, contains the neurotransmitter GABA. While a great deal of evidence suggests GABA is critical to SCN network synchrony, there is still controversy over exactly what role GABA serves in SCN function. We are using genetic approaches to investigate how GABA affects SCN network properties and circadian behavior in the mouse.
Synaptic Remodeling in the SCN
At the core of circadian rhythms is a transcription/translation feedback loop (TTL) controlled by specific
clock gene products. In the SCN, cells operate together to amplify these signals in concert, driving neuronal circuits which inform the behavior of the organism. These emergent and hierarchical physiological properties arise via poorly understood processes involving the activities of clusters of neurons and their neighboring glia. Recent evidence supports the notion that there is circadian structural remodeling in the terminals of pacemaker neurons. In collaboration with Fernanda Ceriani at the Fundación Instituto Leloir, we are investigating circadian changes of synaptic structure in the SCN and hypothesize that these changes play an important role in the central timekeeping mechanism.
Sleep in Young Adults Experiencing Homelessness
Homelessness is currently one of the largest public health crises facing the Seattle region, and has a range of complex causes from lack of affordable housing to health disparities. We use wrist actigraphy to chronically monitor sleep in young adults experiencing homelessness in Seattle, and study the relationship between lack of permanent housing, sleep quality and other health outcomes. We are also conducting a series of interviews with our study participants, through which we aim to both highlight their experiences and bring greater public awareness to sleep health disparities in homeless populations.
Novel Analysis Methods for Circadian and Sleep Data

The study of circadian rhythms requires investigators to conduct months-long experiments, generating large and often unwieldy time series data. This problem is especially prevalent in the study of the circadian regulation of sleep, which requires long-term continuous recordings of electroencephalography (EEG) and electromyography (EMG) signals, and produces data that is laborious and error-prone to analyze. Surprisingly, few reliable software packages for the automatically scoring sleep stages exist, and the ones that do are often expensive or unintuitive for researchers without computational skills. In collaboration with Bing Brunton at UW, we are using machine learning to develop novel methods for automatic scoring of sleep stages and seizure detection in rodent studies of sleep. Our goal is to make our software freely available to the circadian and sleep communities so that we can empower more researchers to study the circadian regulation of sleep in health and disease.