
Old issues - new infections
- Limited Response Capacity = Limited incentive for monitoring infections
- Access issues compromise diagnostics
- Technology can help but not assure success
- New insight, strategies are needed
Limited capacity = limited incentive
Some old issues in surveillance are pertinent to new infections. First of all, if you have a limited response capacity to something, youíre going to have a limited incentive to count it. An excellent example is meningitis that occurs regularly as major epidemics in sub-Sahara Africa. This is the "Meningitis belt," and people in the region tell you itís because of the dry, dusty "Hamartan" winds that come off the desert:. Meningitis kills hundreds if not thousands of people in each country. If you look in the international literature, several people published a report that showed the cyclical, absolutely predictable periodicity and the level of the epidemic. Last year was an epidemic year. The access to vaccine, which is effective, is very limited. One year CDC vaccinated before the anticipated epidemic. This successfully prevented the problem. The next year we didnít have access vaccines and we had a huge epidemic. The point..here is a preventable problem that the countries don't have the resources to prevent. If you donít have anything that you can do about something, your incentive for going out and counting really goes down. Even though it was predictable, there was no response.
There are other examples where because there is no capacity to respond there is no incentive to count. Reemergence of Tuberculosisóthereís no adequate treatment many places. They have active TB cases and know theyíre not being treated. In Indonesia, for example, they know with the number of doses that theyíve given out for Tuberculosis cases that theyíve treated about half of them that they know of, even through their surveillance system. An absolute lack of ability to cope with a problem mitigates against an accurate counting situation. HIV transmission in blood: we know that less than 50% of blood is screened in many countries, about half the countries in the world right now. Dengue and hemorrhagic fever, vector control; Hanta virus, rodent control, Cholera, sanitation and water. Basically the message isnít too complicated.
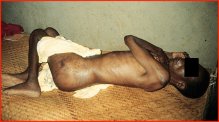
Here is a patient in the Central African Republic who has tuberculosis in his hip. He has already been to the central hospital in Bangui and is on his way back home. He probably has HIV, but during the time he was in the hospital, nobody ever checked if he had HIV. But this is an example of where it may have been as much a question of learning to look or choosing to look. Why compound this fellowís difficulties by giving him a stigmatized diagnosis of HIV, since itís not going to change whatís going to happen to him? So the surveillance cycle can really only apply when you can disseminate use the information effectively in treatment and control, and otherwise there is no reason to count it.

Access
Access issues certainly compromise diagnostics. The biggest delay in international reporting is laboratory delay. Most people will not report a disease internationally until they have confirmed the diagnosis. That can be problematic. If youíre dealing with something unknown, youíre never going to confirm the diagnosis for it. Especially if the unknown is virulent. Also since diagnostics take a long time, youíre 2 or 3 weeks out from the index case of something thatís perhaps highly infectious before you can react.
The World Bank has published this data on access to diagnosis. Although it would be ideal if you had access to laboratory diagnoses, the "surrogate," if you will, is access to the diagnoses of health professionals (nurses, midwives, physicians). These data are highly variable for a number of reasons which include training of the health professionals and patient access to them. Note the number of physicians per thousand population in sub-Saharan Africa (about 0.1 physician per thousand) compared to the established market economies and former socialist economies. There is dramatic difference in access to diagnosis around the globe. This should cause one to pause when you look at reported information on disease.
We know that only 30% of deaths are actually medically diagnosed. Even most deaths donít come to the attention of physicians. Case definition differs.
Characteristics of Internationally Used Case Definitions of AIDS

