|
|
| M A T E R I A L S C I E N C E & E N G I N E E R I N G |
||||||||||||
|
|
|
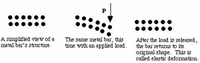
Material Classes Metals Metals are opaque and lustrous (or shiny) elements that are good conductors of heat and electricity. Most metals are malleable and ductile, and are generally more dense than the other pure solid material. When something is malleable, it means that they can be molded. Ductile means that the material can be stretched into a thin wire.
What is their structure? As with all elements, metals are composed of atoms. Let us investigate what each property tells us in the atomic level:
A reasonable model would be one in which atoms are held together by strong, but delocalized, bonds. Please see metallic bonding.
|
|||||||||
| Copyright © 2006 CES Information Guide - Materials Science Engineering |
||||||||||||