- making dilutions
- reading the meter on a simple homemade spectrophotometer
- keeping careful laboratory records
- graphing on linear graph paper
- determining an unknown concentration from known
- using a colored filter to enhance contrast and sensitivity
Timing
- one 45-50 minute class period to build spectrophotometer (if used)
- one 45-50 minute class period for lesson
Background
See also Simple Dilution 2.
Dilution usually refers to a concentration of a material in a liquid and sometimes in a gas. Am I getting my moneys worth? Is it safe to use at this concentration? How much do I need to dilute before use? When we swallow a vitamin C tablet at breakfast we are diluting the vitamin C into our entire body. What is the proper amount of vitamin C for my body size? When we add bleach to a wash load or prepare a soap solution to wash our car, we are diluting a material to the proper concentration.
In later lessons we will be diluting a concentrated sperm suspension to fertilize sea urchin eggs. Too much and we prevent proper development, too little and they will not all fertilize. How much do we need to dilute the sperm to get the highest rate of survival of the embryos? In the experiment lab students will be diluting environmental "toxins" to see their effect on fertilization and development. They will need to understand dilution in order to be able to do these experiments in any kind of meaningful way.
In this lesson an attempt has been made to relate dilution to the real world. Scenario: You have purchased a "Coke" at the movie theater. Is it at the right concentration? Have I gotten my moneys worth or have they "watered down" the Coke to save money. What happens when the ice melts? How much more dilute is the Coke at the end?
Using Coke as our material of interest we will be performing a simple linear dilution to form a "standard curve" from which we will quantify our unknown samples (the Coke and ice mixtures). A simple device to measure concentration will be constructed using a light sensor, light source, filter and a meter.
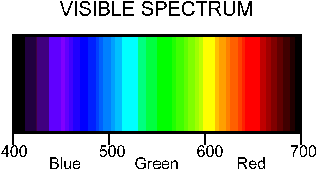
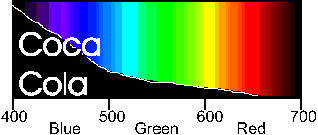
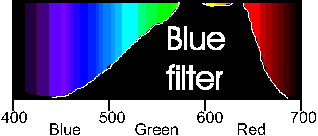
By adding a filter to our light sensor we can improve the "Coke" detecting ability of our sensor. Since Coke absorbs light in the blue we can get the most response from our sensor if we choose a filter that lets primarily blue light through. An inexpensive blue filter's spectrum looks like this:
Materials
- "Classic Coke" - one can per lab group (They will sample it I'm sure.)
- ice in a Styrofoam container.
- water to use for dilutions
- graduated cylinder (10 ml will work fine.)
- clean test tubes (16x100 work great), 8 for each lab group
Spectrophotometer parts: (The "spec" has the potential of being shared with the chemistry classes)
- pkg of 5 photocells - Radioshack #276-1657, $2.29/pkg
- digital multimeter - Radioshack #22-166, $39.99 ea for student lab groups and you may want the Radioshack #22-168 at $129.99 ea for instructor use. This unit will interface with a computer that can then be projected onto a television with the proper adapter.
- black foam core board from an art supply store
- black tape - Black 3M #235 photographer's tape works best, but duct tape will also work.
- blue filter - We suggest the filter pack from Edmund Scientific #A60,373 $16.75. This will give plenty of material to be cut up for class use in this lessons and others.
- light source - It can be a microscope lamp, large flashlight, or direct sunlight.
Procedure
Setting Up the Spectophotometer:
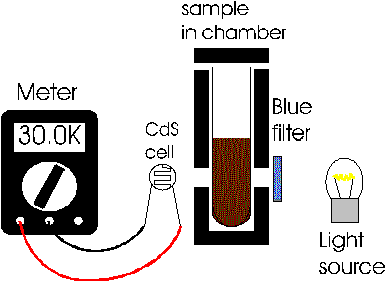
Standards Series:
| % "Coke" | 100 | 80 | 60 | 40 | 20 | 0 |
| ml of Coke | 10 | 8 | 6 | 4 | 2 | 0 |
| ml water | 0 | 2 | 4 | 6 | 8 | 10 |
| total liquid | 10 | 10 | 10 | 10 | 10 | 10 |
Unknowns:
Unknowns can be made up ahead of time or have students devise criteria. Examples:
- Add 100ml of coke and 50 grams of crushed ice to different containers (glass, Styrofoam, paper). take 10ml samples at set time intervals
- Add differing amounts of ice to set amount of Coke and heat to melt ice completely.
- bring back samples of "Cokes" from 7-11, movie theaters, etc. plus/minus ice.
Math
Plot Readings vs concentration: (example shown)
| % Coke | K-Ohms |
| 100 | 3.0 |
| 80 | 2.8 |
| 60 | 2.6 |
| 40 | 2.4 |
| 20 | 2.0 |
| 0 | 1.7 |
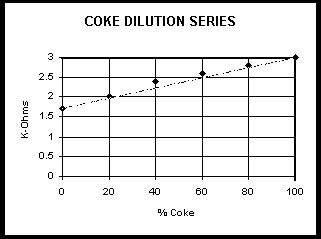
Students may have some trouble with a graph that is not zero K-Ohms at zero Coke concentration. One way around this is to do a "blank subtraction". Just remember to subtract the blank from the unknowns also.
Implications
- This lesson did a linear dilution. How would you do a serial dilution based on a factor of 2? 10? (starting from the same 100% solution).
- In later lessons you will dilute sperm in a similar manner. How could you relate your optical density readings (the K-Ohms reading from the meter) with concentration? (Hint: use an hemacytometer to count sperm at a known dilution.)
- What about a solution that changed color with time. How would you record the results? [K-Ohms on Y axis and time on the X axis].
- How much do you weigh? in grams? (453 grams per pound) If you took a 1000 milligram vitamin C pill (1000 milligrams = 1 gram), how dilute would it be in your body? (assuming an even distribution)
- What would happen if the samples were not mixed well? How would this affect readings?
Evaluation
Class participation and lab report. On the lab report look for the following:
- neatness and well laid out (objective, materials & methods, etc.)
- table of data collected for series
- graph of series
- unknowns and explanation of how values were found
- answers to any Implication questions assigned.
