For those who can recall the devastation brought on by the HIV epidemic of the ’80s, the treatments available today are nothing short of a miracle.
This is especially true for the LGBTQ community – particularly gay men – who faced higher infection rates, widespread stigma and systemic discrimination, hindering access to healthcare and crucial community support as the virus ravaged an already marginalized community.
Despite the progress we’ve made over the last four decades, the AIDS crisis of the 1980s leaves a powerful legacy and remains a crucial part of LGBTQ history and health equity discussions today. 🩺
As another Pride Month comes to a close, we celebrate the progress we’ve made and acknowledge the work still ahead. 🏳️🌈 Turns out, the University of Washington is a fabulous place to start. 💛 🐾 💜
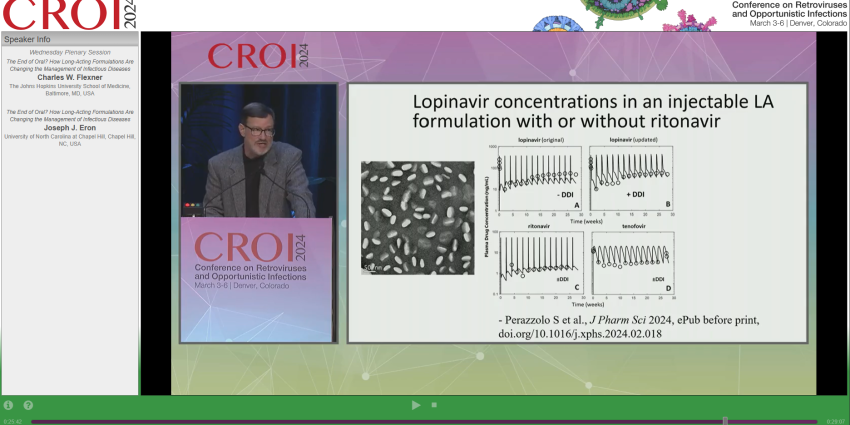
In a recent editorial from The New York Times, scientists at the UW School of Pharmacy are closing in on longer-acting modalities for HIV treatment, like a weekly pill or a monthly shot. 💊
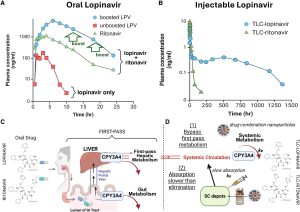
Rooted in advancements from the Department of Pharmaceutics, the researchers behind our Targeted Long-Acting Combination AntiRetroviral Therapy (TLC-ART) program represent a wealth of expertise as they develop therapeutic treatments for HIV, cancer and other infectious diseases.
Long-acting alternatives to traditional HIV treatment have the potential to turn a formidable disease into a manageable diagnosis with encouraging treatment options, especially for patients with inconsistent access to healthcare, housing and transportation – a promising development in a country where 39 million people live with the disease but only half have it under control.
Looking ahead, researchers at the UW feel optimistic about the future for HIV patients – especially when it comes to stigma surrounding the disease, which is a barrier to treatment in itself.
“That internalized stigma of taking a pill every morning is what prevents them from doing it,” said Dr. Rachel Bender Ignacio, Director of UW Positive Research and a member of TLC-ART leadership team.
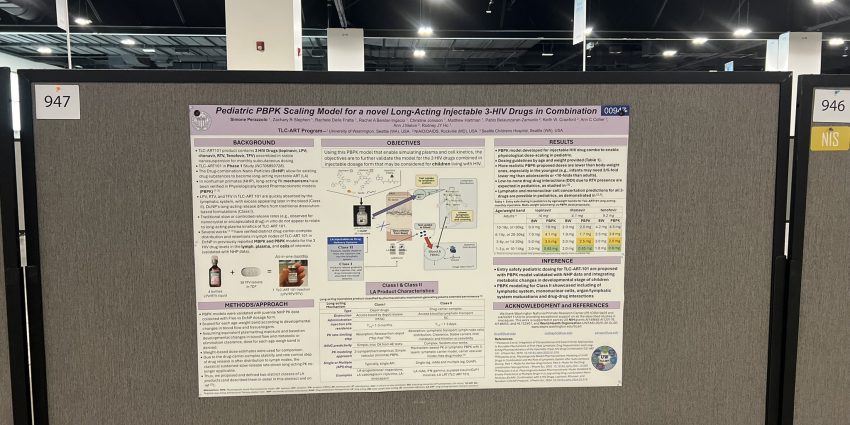
The first phase of clinical testing is currently underway for an injectable, long-acting drug product candidate for HIV developed by the TLC-ART program at UW. Stay updated on their progress: https://lnkd.in/gEXAs4qB
Read the editorial and explore our nod in The New York Times: https://lnkd.in/gd2iW-vX
hashtag#Pharmacology hashtag#Pharmaceutics hashtag#PrescribeMedicine hashtag#PharmaceuticalIndustry hashtag#BiomedicalScience hashtag#Research hashtag#ResearchImpact hashtag#ResearchBreakthroughs hashtag#LabLife hashtag#HigherEdNews hashtag#AcademicCommunity hashtag#ScholarlyWork hashtag#PrideMonth hashtag#HuskyPharmacy hashtag#UWSoP hashtag#UofWA