Dr. Ho and his team attended and contributed to the LEAP workshop and CROI in Denver, highlighting their development in Drug-combination nanoparticle technology. This innovative technology simplifies and scales up the formulation of multiple antiretrovirals into a single injectable for long-acting HIV therpay.
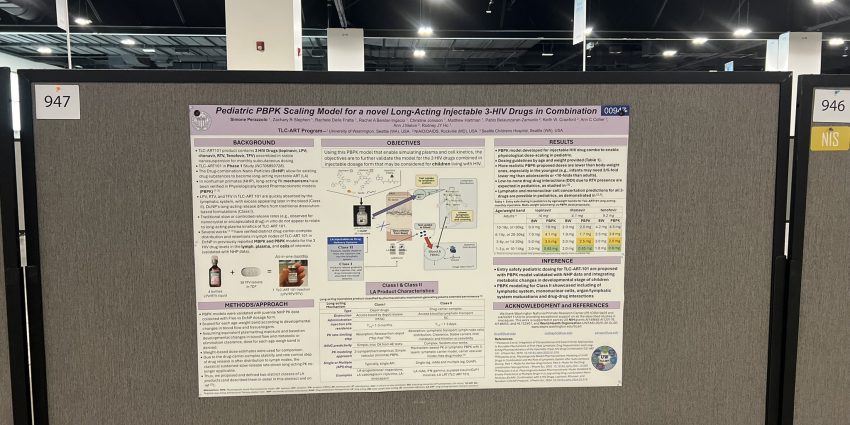
During LEAP, Rodney updated attendees on the project’s progress over the last year. At CROI, their focus was on a poster presentation in the pediatric section, discussing a validated PBPK model for pediatric dosing.
Key topics at CROI included advancements in HIV vaccines, mAbs, long-acting injectables, and pediatric HIV treatment solutions.
For further details, contact tlcart@uw.edu.