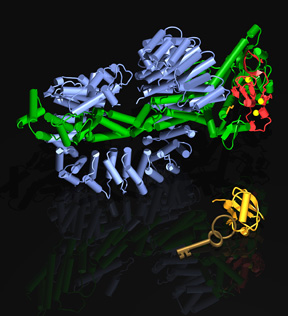
The cullin-RING E3 complexes represent the largest family of multi-subunit ubiquitin ligases in eukaryotic cells. Assembled in a modular mode, cullin-RING E3 machineries are built on a catalytic core consisting of a cullin scaffold protein and a RING finger protein. Humans have six closely related cullins, Cul1, 2, 3, 4A, 4B, and 5. Using a unique adaptor, each cullin scaffold can organize a distinct sub-family of E3 complexes by recruiting a group of substrate receptor subunits. Together, these cullin-RING complexes promote the ubiquitination of many well-known regulatory proteins such as the Wnt signaling protein beta-catenin, the NF-kappaB inhibitor IkappaB, the Cyclin-Cdk inhibitor p27, the oxygen-regulated transcription factor HIF1alpha, the antioxidant response transcription factor Nrf2, and the DNA replication licensing factor Cdt1. In recent years, we have been investigating an emerging subfamily of cullin-RING E3s, the DDB1-CUL4-DCAF complexes.
Regulation of Cullin-RING E3 Complexes
All cullin-RING E3s are regulated by a numer of common cellular factors including the ubiquitin-like cullin modifier Nedd8, the cullin inhibitor protein CAND1, and the eight-subunit protein complex, the COP9 signalosome. How such a network of proteins comes together and regulates the functions of cullin-RING E3s is unclear. We seek to address this question with a combination of structural and biochemical approaches.