
erythropoietin and cancer
Up to 40% of patients with cancer are anemic at the time of diagnosis, and a large body of evidence indicates that anemia is a key predictor of survival, independent of disease severity. The mechanism whereby anemia impairs outcome in patients with cancer is not well understood, but has been attributed in part to tumor hypoxia, which can select for tumor cells resistant to chemotherapy and radiation therapy. This rationale helped to lay the groundwork for using Epo for the treatment of anemia in patients with cancer. Recombinant Epo first received FDA approval for the treatment of cancer-related anemia in 1993, and subsequently grew to become the most commercially successful drug in all of oncology. However, the results of Phase III clinical trials now indicate that Epo can reduce survival rates and promote tumor progression in cancers of the head and neck, breast and in most common forms of lung cancer. Although some of these trials are not yet published, and several of the published trials have been criticized for design limitations, the FDA issued a "black box" warning for Epo in March 2007. How Epo stimulates tumor progression is uncertain, but may reflect off-target effects (figure, below). EpoR transcripts have been detected in multiple primary cancers including tumors of the breast, head and neck, and non-small cell lung. Some reports have documented Epo-induced proliferation, invasion, migration, and protection from chemo- and radio-therapy in various cancer cell lines. Moreover, blocking endogenous Epo can inhibit breast cancer growth and tumor vascularization in a rat model, and ovarian and uterine cancers in mice. Finally, EpoR expression and function in endothelial cells is well-documented, and Epo might stimulate tumor progression by promoting tumor angiogenesis.
Is Erythropoietin Induced Tumor Progression An Off-Target Effect?
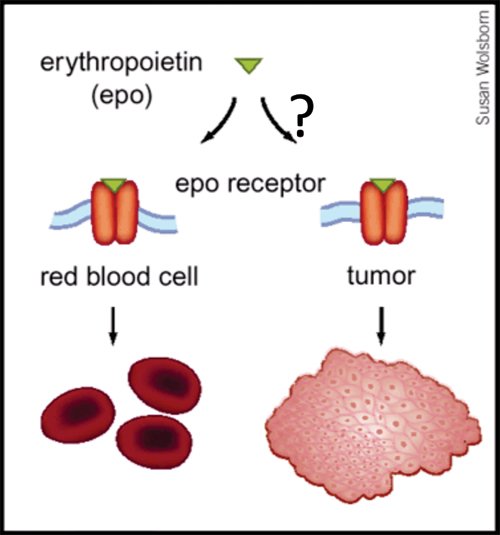
From Nature Medicine, 2003
One of the phase III trials suggesting that Epo might promote cancer progression, was ENHANCE, a trial of 351 patients with head and neck cancer (Henke et al., Lancet 2003). ENHANCE demonstrated a shorter locoregional progression free survival (LPFS) in head and neck cancer patients randomized to Epo rather than placebo during radiotherapy. ENHANCE incorporated patients who underwent complete, partial or no resection of tumor prior to radiotherapy. Adverse effects of Epo were confined to patients with residual tumor at the time of radiotherapy. We developed an assay to measure mRNA levels of genes implicated (EpoR, Jak2, Csf2rb, Hsp70) or not implicated (Krt5, Cd44) in Epo signaling in formalin-fixed paraffin-embedded (FFPE) tumors, and tested 136 tumors from ENHANCE. We compared LPFS between patients with tumors expressing above- versus below-median levels of the aforementioned mRNAs using the log rank statistic. Sufficient RNA for EpoR measurements was available in 101 tumors and EpoR varied over a 30-fold range. There was no association between tumor EpoR level and LPFS across all 101 patients. However in patients with unresected tumors (n=28), above-median EpoR mRNA levels were associated with significantly poorer LPFS if randomized to Epo rather than placebo (p=0.02, n=14). A similar association was observed in patients with above-median levels of Jak2 mRNA (p=0.02, n=18) or below-median levels of Hsp70 (p=0.01, n=20). LPFS was not significantly different when comparing Epo-treated patients with above-median mRNA levels to Epo-treated patients with below-median levels. EpoR mRNA levels can be reliably measured in FFPE tumors. These associations merit evaluation in larger numbers of tumors from other Phase III trials.
Papers
Blau CA. Erythropoietin in Cancer: Presumption of Innocence? Stem Cells 2007; 25:2094-7.
Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kunzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous Thromboembolism and Mortality Associated with Recombinant Erythropoietin and Darbepoietin Administration for the Treatment of Cancer-Associated Anemia. JAMA 2008;299: 914-924.
Miller CP, Lowe KA, Valliant-Saunders K, Kaiser JF, Mattern D, Urban N, Henke M, Blau CA. Evaluating Erythropoietin-Associated Tumor Progression Using Archival Tissues from a Phase III Clinical Trial. Stem Cells 27:2353-61, 2009.
Miller CP, Valliant-Saunders K, Blau CA. Limitations of a murine transgenic breast cancer model for studies of erythropoietin-induced tumor progression. Translational Oncology 2010 Jun 1;3(3):176-80.
Miller CP, Urban N, Blau CA. Quantitative Comparison of Erythropoietin Receptor Levels in the Epithelial versus Endothelial Fractions of Primary Breast Tumors. Anticancer Res. 2011 Apr;31(4):1189-95.